Environmental Chemistry/Soil
Soil is a topic for Environmental Chemistry for the 2009 and 2010 seasons.
The Competition
The 2009 event is a lab and testing event with teams of two people. The test consists of questions on basic knowledge of soil, minerals, soil chemistry, soil horizons, and may be taken in station format or a single written test taken at one table. Experiments may range from testing the pH of a soil sample or the amount of either one of the components in NPK in that same sample, and making recommendations of what fertilizer should be added, density of soil particles, and so on and so forth.
Parameters
Each student may bring a non-programable calculator and a pencil. Each team may bring a two-sided 8.5" by 11" note sheet with any information in any form. Students must also have Z87 safety goggles, pants that go down to the ankles, and a chemical apron.
Suggestions
Spend some time practicing testing soils for properties such as pH, N-P-K content, and moisture. You can find simple soil testing kits at Lowes or Wal-mart.
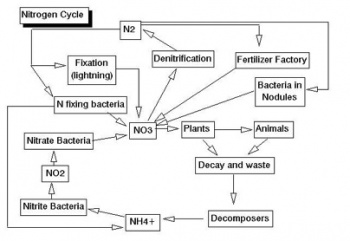
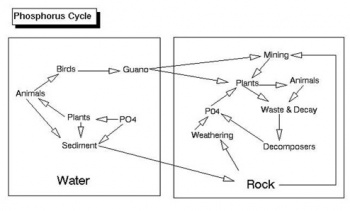
You should know how particular nutrients help the plants. For example, nitrogen promotes healthy, green leaves and growth of a plant. Know the major nutrients in soil (nitrogen, phosphorus, potassium), the secondary nutrients (calcium, magnesium, sulfur), as well as micronutrients (such as iron, manganese, zinc, boron, copper, molybdenum, and chlorine). Also, know how they help the plant (they all help in at least one way). Have an understanding of the different cycles (nitrogen cycle, phosphorus cycle, sulfur cycle), and know about substances that can be added to a soil to alter pH and other properties.
Plants
Environmental Chemistry has some plant questions as well, including pH and other growth concepts.
Nutrient Functions
- Nitrogen: promotes healthy green leaves and fast growth (chlorophyll, cell formation, proteins/amino acids)
- Phosphorus: used for roots, seeds, fruit, flowers, fighting disease and fast growth (cell formation, protein synthesis, metabolism)
- Potassium: strong stems, fighting disease and fast growth (water regulation, enzyme activity)
- Calcium: root absorption, growth regulating enzymes, cell wall formation and cell division, disease resistance (enzyme activity, root permeability)
- Magnesium: component in chlorophyll, phosphorus carrier (chlorophyll, metabolism)
- Sulfur: synthesis of certain amino acids, photosynthesis, winter hardiness (formation of oil, vitamin, protein and amino acids)
- Iron: chlorophyll formation, carrying oxygen (enzyme activity and development)
- Manganese: metabolism, photosynthesis (pigmentation, enzyme activity)
- Zinc: growth regulation, metabolism (enzyme activity)
- Boron: cell wall formation and rapid growing points (enzyme activity)
- Copper: activates enzymes, growth (enzyme activity)
- Molybdenum: synthesis and activity of nitrate reductase, symbiotic nitrogen fixation in legumes (enzyme activity, nitrogen fixation in legumes)
- Chlorine: energy reactions, transport of nutrients (chlorophyll formation, cellular development, enzyme activity)
Plant Signs
- Yellowing of leaves: upper leaves lack sulfur, lower leaves lack nitrogen
- Yellow spots on leaves: upper leaves lack iron, lower leaves lack magnesium
- Too much unnatural dark green on leaves: lack of phosphorus, too much nitrogen, possibly too much phosphorus
- Blackening of leaves' edges: lack of calcium
- Yellowing of leaves in between veins: too much iron, too much manganese, possibly too much magnesium, possibly too much potassium
Soil
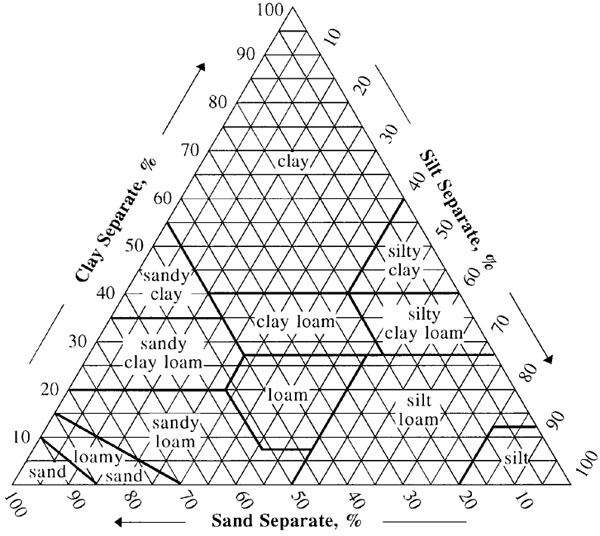
Soil Texture
You will need to know how to read a soil texture triangle. Remember that percentage of clay is read on the horizontal lines, left to right, the percentage of sand is read from bottom right to top left, and the percentage of silt is read from top right to bottom left.
You will also need to know the size of the soil separates:
- Sand: 2.0 mm to 0.02 mm.
- Silt: 0.02 mm to 0.002 mm.
- Clay: 0.002 mm and finer.
Soil Contaminants
- Lead (Pb)
- Nickel (Ni)
- Mercury (Hg)
- Cadmium (Cd)
- Chromium (Cr)
- Other Heavy Metals
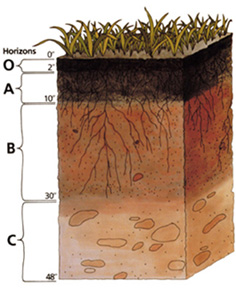
Soil Horizons
Some things you may want to be familiar with are the different soil horizons. The main horizons are shown in the diagram below.
- O: Organic matter. Or "litter layer." This generally consists of decomposing plant matter.
- A: Surface Soil. Or topsoil. This is where plants will grow and obtain nutrients
- B: Subsoil. This layer accumulates Iron, clay, Aluminum, and organic compounds through a process called illuviation.
- C: Substratum. Accumulates more soluble compounds that bypass layer B.
Cation Exchange Capacity (CEC)
CEC is defined as the total amount of positive ions (cations) that a soil can absorb exchangeably. It is commonly reported in milliequivalents/100g dry soil. Basically, in a soil there are positively and negatively charged nutrients. Positive charges lie on the top, for the plant to absorb. When all are used up, more cations must be added to "fill the void" so to speak. If the soil has a higher CEC, it can absorb more cationic nutrients, making them available to plants (nutrients that just lie on top of the soil are useless to the plant). Soil containing high clay or organic matter will have a high CEC.
Soil Remediation
Soil remediation is the process of decontaminating and revitalizing soil.
- Low-Temperature Thermal Desorption: also known as LTTD, thermal stripping or soil roasting. Uses heat (<100°C) to separate pollutants (petroleum hydrocarbons, ex gasoline or jet fuel, etc) from soil. Heat can be applied with a rotary dryer, asphalt plant aggregate dryer, thermal screw or conveyor furnace. Once separated, vaporized pollutants are either treated in secondary treatment units before being discharged into the atmosphere or collected through condensation. Effective in all types of soil. Ex-situ.
- Stabilization & Solidification: also known as S/S. Contaminants are rendered immobile through reactions with additives or processes. "Stabilization" refers to the process of transforming contaminants into a less mobile form. "Solidification" refers to the process of increasing the solidity and structural integrity of the material. This method DOES NOT get rid of the pollutant, it simply makes it less mobile. This method is best for soils with inorganic compounds, metals or radionuclides. Ex-situ or in-situ.
- In Situ Chemical Oxidation: also known as ISCO. Oxidants (the 4 major oxidants are permanganate, persulfate, hydrogen peroxide and ozone) are pumped into the soil, causing redox reactions that convert contaminants to less toxic, less mobile, less harmful products. In-situ.
Fertilizer
Reading Fertilizer Labels
The key part of fertilizer label reading is finding the quantity of the major nutrients. Somewhere on the bag, there will be a line similar in appearance to #-#-# (N-P-K). These numbers signify the quantity of the major nutrients. It is based on how much of each nutrient would be in a 100 lb. bag. The first number represents the amount of nitrogen, the second represents the amount of phosphorus, and the third represents the amount of potassium. Rarely, there will be a 4th number listed. This number would represent the amount of sulfur. For example, if a 100 lb. bag of fertilizer was labeled 25-5-10, it would contain 25 lbs. of nitrogen, 5 of phosphorous, and 10 of potassium; the remaining 60 pounds are inert or inactive ingredients.
Calculating Percentage
Some questions will deal with percentages of N-P-K. Going back to the example above, if you have 100 lbs. of 25-5-10 fertilizer, there is 25% nitrogen, 5% phosphorus, and 10% potassium. Another example is when the question gives you measurements in moles. Ex: If 100mol of fertilizer contains 25% nitrogen, 30% phosphorus, and 45% potassium, how much nitrogen, phosphorus and potassium is there in grams?
Step 1:
0.25 * 100mol = 25mol of N
0.3 * 100mol = 30mol of P
0.45 * 100mol = 45mol of K
Step 2:
For N: 1mol = 14.01grams
then: 25mol of P = 350.25grams of N
For P: 1mol = 30.97grams
then: 30mol of P = 929.1grams of P
For K: 1mol = 39.10grams
then: 45mol of K = 1,759.5grams of K
Nutrients
Major Nutrients
- Carbon (C): forms the backbone of plant bio-molecules, includes starch and cellulose. It is fixed through photosynthesis.
- Hydrogen (H): essential for the building of sugars and therefore the plant.
- Oxygen (O): necessary for the cellular respiration of the plant; O2 is the by-product of photosynthesis.
- Nitrogen (N): especially important for growth of plants. Without it, plants will have stunted growth. Plants with good amounts of nitrogen will have strong roots and healthy foliage.
- Phosphorus (P): plays an important role in photosynthesis. It helps in the formation of the plant's flower/seed. It also increases resistance to diseases. Without Phosphorus, Nitrogen could not so its job properly
- Potassium (K): increases a plant's drought tolerance because it reduces water loss in leaves. Like phosphorus, it helps resist disease. It is responsible for water use to move nutrients. It assists in the ripening of fruits, especially grains.
Secondary Nutrients
- Sulfur (S)
- Calcium (Ca)
- Magnesium (Mg): Magnesium is important to a plant because it is the central core of the chlorophyll molecule. It also helps with the plants metabolism and without it, the plant's growth becomes stunted.
Minor Nutrients
|
|
Level of Plant Nutrition for each N-P-K
- Heavy Nitrogen: leeks, potatoes, red beet, spinach, summer cabbage, winter cabbage, summer cauliflower
- Moderate Nitrogen: squash, beans, lettuce, onions
- Low Nitrogen: carrots, parsnips, radish, turnips
- No Nitrogen: peas
- Heavy Phosphate: lettuce, spinach
- Moderate Phosphate: carrots, beans, onions, potatoes
- Low Phosphate: Brussels sprouts, peas, radishes, summer cabbage, red beet, turnips, summer cauliflower, leeks, parsnips
- No Phosphate: winter cabbage
- Heavy Potash: spinach
- Moderate Potash: beans, leeks, onions, turnips, potatoes, red beet
- Low Potash: Brussels sprouts, carrots, summer cauliflower, summer cabbage, winter cabbage, lettuce, parsnips, peas, radish
- No Potash: squash
pH
The pH scale is a logarithmic scale that goes from about 1 to 14. 1-6.9 is considered acidic, while 7.1-14 (or sometimes only to 12) is considered basic. A 7 is neutral, like distilled water. Most garden vegetables like slightly acidic soil (5.5-6.8). A pH of 3.2 is the pH of acid rain (sulfuric acid,) What makes things acidic or basic? Hydrogen ions (H+) make soil acidic. While OH- ions make a soil basic.
Determining pH
They are different ways to determine pH. One is using Litmus paper. When Blue Litmus paper is put in an acid, it will turn red. When red Litmus paper is put in a base, it will turn blue. If you use pH paper, here is a way to test your soil:
- Mix your sample of soil with distilled water in a 1:5 ratio of soil to water.
- Shake the mixture up and let it sit.
- Once your mixture has settled, put in the pH paper and wait for a color to show up.
A second way is using pH paper. pH paper can turn a variety of colors when placed in the substance. Each color represents a certain pH. To find out the pH, one would consult a scale. Lighter colors (oranges and reds) are generally acids, while cooler colors (blues and greens) are generally bases.
A third way is using universal indicator liquid. Placing a single drop of the liquid in a substance will turn it a certain color. Again you would consult a scale to determine the pH number.
A fourth way of determining pH is to use either an Analog or Digital pH meter. Simply place it in the soil for a couple minutes and read the pH number.
Altering pH
Certain substances can be added to either raise or lower the pH of a soil.
- To raise pH (make more basic), add limestone, lime
- To lower pH (make more acidic), add sulfur, aluminum sulfate, ammonium sulfate, ammonium nitrate
Pesticides
Chemical Pesticides
- Organophosphate Pesticides - Affect nervous system by disrupting acetylcholine regulator. Toxic, not persistent in environment.
- Carbamate Pesticides - Affect nervous system by disrupting acetylcholine regulator. Reversible, has several subgroups.
- Organochlorine Insecticides - Removed from market due to health and environmental effects/persistence. (Chlorodane, DDT)
- Pyrethoid Pesticides - Synthetic version of pyrethrin (found in chrysanthemums). Modified to increase stability. Can be toxic.
Biopesticides
- Microbial - Microorganism as active ingredient. Commonly Bacillus thuringiensis (Bt).
- Plant Incorporated Protectants - Gene insertion to code for pesticide production, often from Bt. Also called "PIP"
- Biochemical - Non-toxic, naturally occurring. (Pheromones, scents for traps, etc.) Special committee in EPA for classification.
Biogeochemical Cycles
Periodic Table
It is a good idea to be familiar with the Periodic Table of the Elements. You will see the Latin abbreviations of the elements everywhere.
Sample Questions
- Given a fertilizer label of 10-15-10, write the percentage of nitrogen (N), potassium (K), and phosphorus (P).
- Given results of a tested soil, make recommendations of what fertilizers should be added to yield the largest amounts of vegetables, such as corn.
- Rate soil particles from smallest to largest: clay, loam, sand, silt.
- Name two benefits of adding lime to a soil.
- Upon being given three soil samples, identify which is clay, silt and sand.
- If a soil has a pH of 9.0, what should be added to it to obtain an pH of around 6.0–7.0?
- Define anion and cation.
- Nitrogen fixation can also occur due to lightning. What reaction is occurring?
- What does LTTD stand for? What kind of pollutants are targeted by LTTD?
- What element does red soil usually derive its color from?
| Division B: Science Quiz Bowl · Parasitology | Division C: Detector Building · Environmental Chemistry |
| Division B: Aerial Scramble · Amazing Mechatronics · Codebusters · Storm the Castle | Division C: Aerial Scramble · Amazing Mechatronics · Environmental Chemistry · Trajectory |