Food Science
This page is incomplete. |
| Food Science | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Chemistry | ||||||||
| Category | Lab | ||||||||
| Description | Students will answer questions on food chemistry with a focus on sugars. In addition, participants will build a hydrometer capable of measuring sugar solutions between 1-10% (mass/volume). | ||||||||
| Event Information | |||||||||
| Latest Appearance | 2022 | ||||||||
| Forum Threads | |||||||||
| |||||||||
| Question Marathon Threads | |||||||||
| |||||||||
| Additional Resources | |||||||||
| Image Gallery | Link | ||||||||
| Official Resources | |||||||||
| Website | www | ||||||||
| Division B Results | |||||||||
| |||||||||
Food Science is a Division B event for the 2022 season that focuses on candy making. Previously, the topic was fermentation and pickles. The competition includes a test portion on the chemistry of food, with a focus on Candy Making and sugars. There is a lab portion of the event, in which competitors measure the sugar concentration of solution(s).
The Event
The event (2017-2018) consisted of a written test and a lab portion where students must use a homemade calorimeter. The competition often required competitors to run chemical detection tests such as Benedict's test or a Biuret test. The event (2019-2020 and 2020-2021) was focused on fermentation and pickling. It consisted of a lab portion, where you had anywhere from 1 to all of the following labs: using a student-made salinometer to find the salt content (0-10%) of an unknown solution, finding the pH of a pickle, and finding the water content of a pickle. Currently, The event (2021-2022) focuses on sugars. It consisted of a written test and a lab portion, where students use a homemade hydrometer. To be allowed to participate, you must bring:
- ANSI Z87 goggles (eye protection #4)
- Lab coats or lab aprons that reach the knees. If aprons are used, then sleeves must reach the wrists.
- Closed-toe shoes (no sandals)
- Pants or skirts that cover the legs to the ankles (leggings don't count)
- 1 page (front and back) per competitor, containing notes in any form from any source
- Plastic bags, cups, and spoons
- Graduated cylinders and beakers
- pH paper
- A homemade hydrometer
Key Terms
You should memorize these key terms, and understanding these terms is going to be necessary for following the wiki.
Fahrenheit - A degree of measuring temperature. Water boils at 212 Fahrenheit and freezes at 32 Fahrenheit.
ADP - ADP stands for adenosine diphosphate. It is a very common molecule in your body, and is part of DNA.
ATP - ATP stands for adenosine triphosphate. It is ADP + a phosphate group
Phosphate Group - A phosphate group is basically a phosphate atom with 4 oxygen atoms bound to it. It contains some energy, which is why ATP contains more energy than ADP.
NAD+ - NAD+ stands for nicotinamide adenine dinucleotide. This molecule is very important, especially in the electron transport chain.
NADH - NADH is basically the oxidized version of NAD+. If you are confused on how the Hydrogen molecule came to be, a hydrogen molecule is just one electron and one proton. Since we have one proton, if we add an electron, we get a H atom.
FAD - FAD stands for flavin adenine dinucleotide. This molecule is converted to FADH2 in the citric acid cycle(see below).
FADH2 - FADH2 is FAD + two hydrogen molecules. This is produced from FAD in the citric acid cycle. This is converted back to FAD in the Citric acid cycle.
Sugar Stages
Thread Stage:
- 230 to 235 degrees Fahrenheit
- about 80 percent sugar
- Forms threads when dropped in cold water
Soft Ball Stage:
- 235 to 240 degrees Fahrenheit
- about 85 percent sugar
- Forms a soft flexible ball when put in cold water
Firm Ball Stage:
- 245 to 250 degrees Fahrenheit
- about 87 percent sugar
- Forms a firm ball when put in cold water
Hard Ball Stage:
- 250 to 265 degrees Fahrenheit
- about 92 percent sugar
- Forms a hard ball when put in cold water
Soft Crack Stage:
- 270 to 290 degrees Fahrenheit
- about 95 percent sugar
- After put in cold water, you can bend the resulting solid a little, then it breaks.
Hard Crack Stage:
- 300 to 310 degrees Fahrenheit
- about 99 percent sugar
- After dropped in water, if you try to bend it, it breaks
Clear Liquid Stage:
- 320 degrees Fahrenheit
- about 100 percent sugar
- no crystallization happens
Brown Liquid Stage:
- 338 degrees Fahrenheit
- 100 percent sugar
- Caramelization happens
Burnt Liquid Stage:
- 350 degrees Fahrenheit
- 100 percent sugar
- Sugar is burnt at this stage
- The solution is no longer candy, and you can not go past this stage(any higher temperatures is still the Burnt Liquid Stage)
Fermented Foods
Types Of Fermented Foods
ExpandFermentation is no longer part of the rules, starting in the 2021-2022 season
|
Acidified Foods
Standard of Identity
According to Federal Regulations, acidified food is classified as non-acid food with acid added to it. It should have a finished equilibrium pH of 4.6 or below and a water activity (aw) greater than 0.85. A non-acid canned food is a food with that of a finished equilibrium pH of greater than 4.6, with the exception of tomatoes and tomato products, which have a finished equilibrium pH of less than 4.7. Low-acid canned foods also have a water activity (aw) less than 0.85.
Types of Metabolic Pathways
Anabolic Pathway
The anabolic pathway is part of a set of metabolic pathways in which the main job is to use energy (endergonic) synthesize (create) molecules from smaller units, the reverse of the catabolic pathway. The energy used for this is from the catabolic pathway used to break down molecules for cellular respiration, using the ATP produced by the oxidation of certain molecules. Reducing agents are used as cofactors and metal ions in enzymes are used to stabilize charged groups in substrates. The substrates used are usually molecules taken from the catabolic pathway. Some functions of the anabolic pathway are to build and grow tissues and organs from smaller cells.
Catabolic Pathway
The catabolic pathway is part of a set of metabolic pathways, like the anabolic pathway, which breaks down molecules into smaller units. These smaller units can then be oxidized for use in anabolic pathways. Examples of functions of this pathway are breaking down muscle proteins into amino acids for glucogenesis or fat into fatty acids in the body fat.
Amphibolic Pathway
The amphibolic pathway is a combination of the anabolic and catabolic pathways. An example of an amphibolic pathway is the citric acid cycle, which can be used in the synthesis of amino acids, or the pentose phosphate pathway.
Types of Cellular Respiration
Aerobic Respiration
Aerobic means "involving oxygen." Therefore, aerobic respiration is a process involving oxygen, consisting of three metabolic processes to create ATP from glucose. In order, these processes are glycolysis, the Krebs cycle (also known as the tricarboxylic acid cycle (TCA) or the citric acid cycle), and the electron transport chain with chemiosmosis, better known as oxidative phosphorylation.
Aerobic respiration occurs in the mitochondria of eukaryotic cells (our human cells!). Its final electron acceptor is oxygen, and it also produces water and carbon dioxide as byproducts. Theoretically, 36-38 ATP is produced in this process, a lot more than in anaerobic respiration. This is the reason why this process is the preferred pathway when oxygen is available for aerobic and some anaerobic organisms.
It is represented by the following chemical equation:
[math]\ce{ C6H12O6 + 6O2 -> 6CO2 + 6H2O + 36-38 ATP }[/math]
Glycolysis
Glycolysis is a metabolic (catabolic) pathway of both aerobic and anaerobic respiration. There are multiple types of glycolysis, but the Embden–Meyerhof–Parnas (EMP) pathway is the one most focused on in Food Science. It can occur in conditions with or without oxygen and occurs in the cytosol of both prokaryotic and eukaryotic cells.
Its purpose is to extract energy from glucose by converting it into pyruvate, a three-carbon molecule. Technically, this process produces 4 ATP, but because two are needed for the preparatory phase, one of the two phases of this process, only 2 net ATP is produced. During this process, NAD+ is also reduced (gains electrons) and converted into NADH. Mg2+ is used as a cofactor in steps involving glucose and substrate-level phosphorylation, but Mn2+ can also be used.
This metabolic pathway can be represented by the following chemical equation.
[math]\ce{ C6H12O6 + 2NAD^+ + 2Pi + 2 ADP -> 2 pyruvate + 2 H^+ + 2NADH + 2ATP + 2H2O }[/math]
This process can be separated into ten steps. Each step is described below.
Phase I
Step 1: Glucose is converted into glucose 6-phosphate through the addition of a phosphate group taken from ATP and is catalyzed by the enzyme hexokinase. To keep the glucose concentration from getting too high and leaving the transport, 1 molecule of ATP is utilized for this step and converted into ADP. This step is also known as the phosphorylation of glucose.
Step 2: Glucose 6-phosphate can then be converted into fructose 6-phosphate, an isomer of glucose-6-phosphate through the rearrangement of its atoms. The enzyme phosphoglucose isomerase is used and required to carry this reaction out. This reaction is also reversible under the condition that there is a high concentration of fructose 6-phosphate, which is often not the case, so therefore most of the time this reaction is in the forward direction.
Step 3: The end product of step 2, fructose 6-phosphate is converted into fructose 1, 6-biphosphate, also known as the phosphorylation of fructose 6-phosphate. Once again, a phosphate group used from an ATP molecule is added to fructose 6-phosphate to make this reaction occur, converting ATP to ADP also once again. The enzyme used to catalyze this reaction is phosphofructokinase, an enzyme which is limited where ADP concentrations are low, and vice versa.
Step 4: Fructose 1, 6-biphosphate further continues in this pathway and is split into two isomers, glyceraldehyde-3-phosphate, an aldose, and dihydroxyacetone phosphate, a ketose. The enzyme fructose diphosphate aldolase is used for this step.
Step 5: After step 4, only dihydroxyacetone phosphate continues to step 5, which is then converted into the same molecule as the other molecule product of step 4, glyceraldehyde-3-phosphate, catalyzed by the enzyme triosephosphate isomerase, and thus concluding the first phase of glycolysis.
Phase II
Step 6: Unlike ATP, where something was taken away from it, NAD+ is converted to two molecules of NADH through the addition of electrons, which used from glyceraldehyde-3-phosphate, converting glyceraldehyde-3-phosphate to producing 1,3-bisphosphoglycerate after the addition of another phosphate group included, but not from an ATP molecule. This reaction is catalyzed by the enzyme glyceraldehyde 3-phosphate dehydrogenase. However, NADH must be oxidized and converted back into NAD+ again and again for glycolysis to continue, which can be done in conditions with oxygen. Fermentation can be used as a pathway to do so if oxygen is not available. The end product of this pathway is NADH though, and not NAD+.
Step 7: A high-energy phosphate group is transferred from 1,3-bisphosphoglycerate to ADP, catalyzed by the enzyme phosphoglycerate kinase and forming 3-phosphoglycerate and ATP, respectively. This process of a transfer of a phosphate group to ADP from another molecule is also known as substrate-level phosphorylation, which also occurs in the last step.
Step 8: This reaction is catalyzed by the enzyme phosphoglycerate mutase. It occurs during the movement of the phosphate group in 3-phosphoglycerate from the third carbon atom to the second carbon atom, forming 2-phosphoglycerate, which is an isomer of 3-phosphoglycerate.
Step 9: 2-phosphoglycerate is dehydrated in this second to the last step, losing two moles of water in the process and is converted into phosphoenolpyruvate. The enzyme enolase (aka phosphopyruvate hydratase) helps by catalyzing this reaction.
Step 10: Once again, as stated in step 7, a phosphate group is transferred from phosphoenolpyruvate (aka PEP) to an ADP molecule, forming another ATP molecule and converting PEP to pyruvate, marking the last step of glycolysis. The enzyme pyruvate kinase is used to catalyze this reaction and is required for cellular respiration to continue.
Aftermath
Since we're talking about aerobic cellular respiration here, pyruvate is first converted to Acetyl-CoA and then continues with the Krebs cycle and the rest of the cellular respiration. If a cell fails to reach the aftermath of glycolysis, it will die. However, in anaerobic cellular respiration, fermentation can be used as an alternate pathway since there isn't any oxygen involved in the process of anaerobic respiration.
Pyruvate Decarboxylation
Before continuing with carrying out cellular respiration, as stated in the aftermath section of glycolysis, pyruvate must be first converted to Acetyl-CoA in a process called pyruvate decarboxylation. This happens in a three-step process where pyruvate is broken down to do as said above.
The chemical equation of this process is shown below:
[math]\ce{ Pyruvate + NAD^+ + CoA -> Acetyl-CoA + NADH + CO2 + H^+ }[/math]
These steps are shown and explained as follows.
Step 1: A pyruvate molecule with a hydroxyethyl group with two carbon atoms after the removal of a carboxyl group, where one molecule of CO2 is released into the medium surrounding it. This hydroxyethyl group binds to the enzyme pyruvate dehydrogenase. Since two pyruvate molecules are produced from glycolysis, this step occurs twice.
Step 2: This hydroxyethyl group can then be oxidized to an acetyl group through the removal of electrons that are given to a NAD+ molecule and reduces it to NADH. These high-energy electrons in NADH are used to produce more ATP later on.
Step 3: A coenzyme A is transferred to the acetyl group, forming acetyl-CoA, where it is now finally ready to continue in the Krebs cycle and the rest of cellular respiration.
Krebs Cycle
The Krebs cycle, also known as the citric acid cycle (and TCA cycle), is a metabolic pathway that connects the carbohydrate, protein, and fat metabolism and makes up the second part of aerobic cellular respiration, after glycolysis. However, unlike glycolysis, the Krebs cycle can only occur in conditions with oxygen, the reason that it cannot be part of anaerobic respiration. The cycle occurs in the mitochondria matrix of eukaryotic cells and the cytosol of prokaryotic cells. It can be used in synthesizing amino acids, so this process is known as both catabolic and anabolic (amphibolic).
The cycle can also be represented by a chemical equation, just like glycolysis, see below. In the case of some cells, ADP can be used in the place of GDP and ATP can be used in the place of GTP.
[math]\ce{ Acetyl-CoA + 3NAD^+ + FAD + Q + GDP + Pi + 2H2O -> CoA-SH + 3NADH + 3H^+ + QH2 + GTP + 2CO2 }[/math]
The steps that make up this pathway are explained and shown below.
Step 1: This step is a condensation reaction where a four-carbon oxaloacetate molecule is combined with the two-carbon acetyl group from the acetyl-CoA produced in an earlier process, and a sulfhydryl group (-SH) is bounded with the remaining CoA, which diffuses away and becomes acetyl-CoA after binding with another acetyl group. This reaction is irreversible due to this process being highly exergonic. The rate of this process is directly proportional to the amount of ATP available. The enzyme catalyzing this reaction is citrate synthase.
Step 2: Citrate in this step loses and gains an H2O molecule, and is, as a result, converted to isocitrate, an isomer of citrate. The enzyme catalyzing this reaction is aconitase.
Step 3: α-ketoglutarate, a five-carbon molecule, also known as alpha-ketoglutarate is then formed through oxidation of the product of the previous step, isocitrate. Isocitrate loses two electrons here, and these electrons are handed over to NAD+, converting it into NADH and also forming a CO2 molecule as part of the process. Through negative feedback from both NADH and ATP plus a positive effect of ADP, this step is regulated. The enzyme catalyzing this reaction is isocitrate dehydrogenase.
Step 4: Alpha-ketoglutarate is then oxidized to form a succinyl group, and the reactions are once given to a NAD+ molecule, and converting it into NADH, thus marking the second NADH molecule formed as a product of the citric acid cycle. This succinyl group is combined with CoA, forming an unstable molecule, succinyl CoA. The enzyme catalyzing this reaction is α-ketoglutarate dehydrogenase. This step is very similar and often compared to the third step.
Step 5: The CoA in succinyl CoA is substituted for a single phosphate group and converted to succinate, where a single high-energy bond is formed, and depending on the type of cell, forms GTP from GDP or ATP from ADP through substrate-level phosphorylation. The enzyme used to catalyze this reaction is the succinyl-CoA synthetase.
Step 6: Succinate is dehydrated to form fumarate, where two hydrogen atoms are released and transferred to FAD, where its electrons reduce it to FADH2. The FADH2 molecule is attached to these electrons and therefore becomes an electron carrier in the ETC, along with NADH. NAD+ however cannot be converted to NADH because there isn't enough energy in these electrons. The enzyme catalyzing this reaction is succinate dehydrogenase.
Step 7: Water is now added to fumarate in this step, where it forms malate through hydrolysis, another four-carbon molecule like fumarate. The enzyme catalyzing this reaction is fumarase.
Step 8: This last step is the reason why this pathway is considered a cycle. The product in this step, oxaloacetate can be used in step one once again after another molecule of acetyl-CoA. Now, to the last step. Malate is oxidized to form oxaloacetate and these high-energy electrons are transferred to a NAD+ molecule, converting it into NADH and marks the third and final NADH molecule formed as a product.
Aftermath
After the citric acid cycle, the NADH and FADH2 molecules formed as products and continue to the ETC, where they are used as electron carriers to generate more ATP. Since 2 molecules of acetyl-CoA are produced from one molecule of pyruvate that enters the citric acid cycle, we multiply all the products of the citric acid cycle by two. Also, carbon dioxide does not contain the carbons from acetyl-CoA, even though carbon dioxide is released as part of the singular turn of the citric acid cycle. These two acetyl carbon molecules will be later released in later turns of the cycle and fully incorporated into carbon dioxide.
Oxidative Phosphorylation
Oxidative phosphorylation is simply the combination of the electron transport chain, also sometimes called the electron transport system, and chemiosmosis, the utilization of ATP synthase to generate ATP from ADP. This process can occur in both eukaryotic and prokaryotic cells, but unlike eukaryotic cells, prokaryotic cells do not have mitochondria, so the plasma membrane is used as a location for this process to occur instead.
The chemical reaction of this pathway is as follows. Keep in mind that this equation only includes the ATP synthesized from the utilization of the NADH and FADH2 molecules produced from the citric acid cycle.
[math]\ce{ 24 ADP + 24 Pi + 6 NADH + 2 FADH2 + \frac{1}{2}O2 + 2H^+ -> 24 ATP + 6 NAD^+ + 2 FAD + 2H2O }[/math]
Electron Transport Chain
The electron transport chain, ETC, is a chain of enzymes that transport electrons across the inner mitochondrial membrane, hence the name electron transport chain. Also known as the respiratory chain, it is the last part of aerobic respiration and requires oxygen. The components that are part of the ETC are located in the inner mitochondrial membrane, where the different multi-protein complexes are located, named I to IV. The coenzyme Q and cytochrome C are electron carriers that help to connect these complexes. When the complexes are supercharged enough by the electrons they receive, they pump hydrogen ions across the intermembrane space, the area between the outer mitochondrial membrane and the inner mitochondrial membrane, specifically complexes I, III, and IV. The generation of ATP from the hydrogen ions produced is not part of the ETC, but instead part of chemiosmosis, also known as ATP synthase.
Complex I
Also known as the NADH dehydrogenase complex or NADH-CoQ oxidoreductase, the complex I consists of an L-shaped flavoprotein (fP), where the vertical leg of the protein is in the mitochondrial matrix and the horizontal leg in the inner mitochondrial membrane, that consists of FMN as a prosthetic group, and an iron-sulfur protein, also called Fe-S. NADH first transfers two electrons to FMN, which transfers it to the iron-sulfur protein Fe-S, which transfers it to CoQ. NADH is oxidized to NAD+, FMN is reduced to FMNH2, and Fe-S can be reduced to Fe (ii) or Fe (iii) depending on the energy of the electrons. There is enough energy in the electrons that the complex becomes supercharged and pumps 4 hydrogen ions into the intermembrane space.
Complex II
In Complex II, also known as succinate-CoQ reductase or succinate dehydrogenase, succinate is first oxidized to fumarate and passes on two electrons to FAD, reducing it to FADH2, passing it on to the iron-sulfur proteins, Fe-S (it is not reduced to a lack of energy), which passes it on to CoQ. There is not enough energy in these electrons to supercharge this complex and therefore it cannot pump hydrogen ions (protons) into the intermembrane space.
Coenzyme Q
Coenzyme Q or ubiquinone serves as an electron carrier in the ETC that connects complexes II and II, receives the electrons from complex I and II, is reduced to QH2, and passes these electrons on to complex III.
Complex III
Complex III is also recognized by other names such as cytochrome C reductase or Q-cytochrome C oxidoreductase. The complex starts with two molecules of cytochrome B, receiving the electrons from CoQ and passing them on to the iron-sulfur proteins (once again can be reduced to Fe (ii) or Fe (iii) depending on the energy of the electrons), which pass them on to cytochrome C1, and finally onto cytochrome C. The complex is supercharged like complex I and pumps 4 hydrogen ions from the matrix of the mitochondria into the intermembrane space.
Cytochrome C
Like Coenzyme Q, cytochrome C is also an electron carrier that receives the electrons from complex III that connects complexes III and IV and passes them on to complex IV.
Complex IV
At the start of complex IV (aka cytochrome c oxidase), the last complex of the ETC, copper ions of the A-type (CuA) receives the electrons from cytochrome C and transfers them to cytochrome A. Cytochrome A transfers the electrons to copper ions of the B-type (CuB), which then transports them to cytochrome A3, leading to the final step of complex IV and the ETC, where the electrons are transferred to the final electron acceptor, oxygen, which splits into two half oxygen ions. These ions bond with two hydrogen ions each to form two H2O molecules.
Aftermath
From proton pumps from the energy that supercharges complexes I, III, and IV, a proton gradient is formed in the intermembrane space. There is a high proton concentration in the intermembrane space in comparison to the mitochondrial matrix, where there is a low proton concentration. These protons cannot cross the inner mitochondrial membrane to the matrix because the membrane is non-permeable to these hydrogen ions, basically meaning they will not let them pass through and thus forming an electrochemical gradient.
Chemiosmosis
The proton gradient of hydrogen ions "want" to cross the inner mitochondrial membrane to the matrix, but cannot as said before, because of the non-permeability of the IMM that disallows this. This is what the enzyme ATP synthase is for. Chemiosmosis is also called ATP synthase, after the enzyme used in this process and in some cases referred to as the "fifth complex" of the ETC since it uses the energy from the redox reactions of the ETC to use phosphorylation to convert ADP and inorganic phosphate (Pi) to ATP. The type of phosphorylation that is used in this process is called oxidative phosphorylation.
Overview of ATP Synthase
ATP synthase is an enzyme shaped like a lollipop, is sometimes called F1-Fo ATPase and used for ATP synthesis, which is regulated by concentrations of ADP and ATP, The two units that make up this enzyme are named F1 and Fo. These units are connected by a protein stalk. The following sections will be talking about these units, the different subunits, and their roles in the unit.
F1 unit
The F1 unit is located in the matrix of the mitochondria and is made up of 9 subunits (polypeptide chains): 3 alpha (α), 3 beta (β), 1 gamma (γ), 1 delta (δ), and 1 epsilon (ε). It is water-soluble and is therefore responsible for the hydrolysis of ATP molecules. The alpha and beta subunits make a hexamer that has 6 binding sites, 3 (the beta subunits) of which catalyze the reaction of synthesis of ATP and the other 3 (the alpha subunits) bind ADP and ATP molecules. The gamma, delta, and epsilon subunits make a central stalk (axle), part of a rotating mechanism, acting as a rotor used in the ATP synthesis process. The gamma subunit penetrates the beta subunits, engaging them and allowing them to go through their three conformational changes, loose, tight, and open while synthesizing and releasing ATP.
The conformational changes occur in the order from left to right in the previous paragraph: loose, tight, and then the open conformational change. During the loose conformational change, ADP and inorganic phosphate (Pi) substrates are allowed to bind together loosely, but the reaction that synthesizes ATP is not catalyzed until the tight change. In the tight conformational change, ATP is now produced because the reaction is now catalyzed, binding ADP and Pi more tightly together. Finally, in the open conformational change, the ATP molecules are released and can be used for normal cell activity. A change in conformational change occurs each time the gamma subunit of the central stalk rotates by 120°.
Fo unit
The Fo unit is located in the inner mitochondrial membrane, is not water-soluble, unlike the F1 unit, and is composed of a varying number of subunits depending on the cell. These subunits include a, b, and c for most cells. In human cells, there are six additional types of subunits, including d, e, f, g, F6, and 8 (also called A6L). There is 1 a subunit, 2 b subunits, and 10-15 subunits. The "o" in Fo stands for oligomycin, the inhibitor of the Fo unit. This protein unit allows the protons from the proton gradient created by the electron transport chain to enter through a pore. The c subunits make a c-ring that acts as a rotor which is embedded in the inner membrane of the mitochondria and turns when protons to translocate through the membrane through two half-channels: an entry and exit half-channel.
Aftermath
We see that after the process of the glycolysis and the citric acid cycle, along with the decarboxylation of pyruvate, there are 10 NADH molecules and 2 FADH2 molecules that are used as electron acceptors in the ETC to pump protons and utilize them to convert ADP to ATP. 112 total hydrogen ions are pumped into the intermembrane space, and for every 4 hydrogen ions, one ATP molecule is formed, forming a total of 32 ATP molecules from the NADH and FADH2 molecules and the 4 ATPs (or 2 ATPs and 2 GTPs, which is energy equivalent to 4 ATPs) produced from glycolysis and the citric acid cycle. While it is said that 38 ATP molecules are produced in aerobic respiration and the total number of ATP molecules total to 38 using the chemical equations, this is only based on theory and does not account for the energy required to move molecules to the mitochondria. Therefore, only 32 ATP molecules are produced from aerobic respiration. This is also only if the malate-aspartate shuttle is used as well and not the less-efficient glycerol-phosphate shuttle is used, which only produces 30 ATP molecules. The following paragraphs will briefly explain what these two are and why they produce the number of ATP molecules that they do, but will not go too in-depth.
Firstly, both are used as a transportation method to transport the molecules of NADH produced from glycolysis to the mitochondria from the cytosol. In the malate-aspartate shuttle, the electrons from the NADH molecules are first donated to oxaloacetate but are eventually given back to the NADH molecules, transferring them back from NAD+ molecules and can continue to the electron transport chain where they will be used in the first complex as electron acceptors and donors. Contradicting this, the NADH molecules produced from glycolysis when using the glycerol-phosphate shuttle are first converted to FADH2 and therefore bypassing complex I in the electron transport chain onto complex II serving the same role and purpose, but only produces 1.5 ATP in ATP synthesis for each molecule as compared to the 2.5 produced for each NADH molecule.
Anaerobic Respiration
Unlike aerobic respiration, which uses oxygen as its final electron acceptor, anaerobic respiration does not require oxygen. There are two methods of anaerobic respiration, one which uses an organic molecule such as pyruvate normally as its final electron acceptor, commonly just referred to as fermentation, and one that uses an inorganic molecule as its final electron acceptor, this one named and known as anaerobic cellular respiration. The second method where an inorganic molecule is used as the final electron acceptor produces more ATP than the first, making it better as a way to obtain energy through ATP for organisms that don't use oxygen rather than just a "just in case" kind of pathway.
The second method, in theory, can produce 5-36 ATP whereas the first method only produces 2 ATP through glycolysis. Anaerobic organisms use the second method as a way of producing ATP without oxygen as said before, and the first fermentation method is used more by bacteria and yeast cells to ferment food or in human muscle cells as an example when there is a lack of oxygen to undergo aerobic cellular respiration.
Method I
The first method starts off with glycolysis and pyruvate decarboxylation, the exact same way in aerobic respiration, except that the NADH molecules are never used in the ETC since it doesn't exist in this pathway. Instead, the NADH molecules are converted to NAD+ through either ethanol or lactic acid fermentation, with the addition of pyruvate being converted to lactate or ethanol, dependent on the organism. More information about these two can be found in the Types of Fermentation section.
Method II
The stages of the second method are relatively similar to aerobic respiration, except that an inorganic molecule is used, as said before. This molecule has a lower reduction potential than oxygen, though this reduction potential varies among the inorganic molecules used, and this makes the amount of energy (ATP) produced in ATP synthesis smaller. The type of inorganic molecule also is dependent on the type of organism itself and can change how much ATP is produced, and this is why that in theory, not accounting for the energy used to transport molecules, 5-36 ATP molecules can be produced.
Fermentation
Fermentation is a metabolic process where organic molecules, such as carbohydrates, are broken down chemically by a microorganism such as yeast, and in some cases converting it to an acid or alcohol through enzyme action anaerobically. One of its goals is to regenerate NAD+ from NADH so glycolysis again. It can also be used as a method of food preservation, used in ancient times. Microbiologist Louis Pasteur discovered that fermentation can be carried out by yeasts and other organisms. The science of fermentation is also known as zymology, studied by scientists called zymologists. According to the rules, the three types of fermentation processes that must be studied for this event is alcohol (ethanol) fermentation, heterolatic fermentation, and homolactic fermentation. The mechanic of how each one works is explained in the following sections.
Health Benefits
There are many health benefits of fermentation. Some include the increase of shelf-life, an improved taste of food, food preservation, as well as having anti-microbial effects. Others include the promotion of probiotics that balance good gut bacteria, help stop digestive problems, add beneficial vitamins and minerals, boosting the immune system, destroying antinutrients that interfere with nutrient absorption, and breaking down nutrients for easier digestion. More benefits that could potentially occur but are not guaranteed to include lowering blood pressure and LDL levels, reduction of depression and anxiety, and weight loss. However, some side effects of eating too many fermented foods are bloating and large amounts of gas.
Types of Fermentation
Alcohol Fermentation
Alcohol fermentation, also called ethanol fermentation, is used in many alcoholic beverages such as beer and wine, where ethanol is the intoxicating agent used. Alcohol fermentation is also used to make bread dough rise through the carbon dioxide produced from the reaction. Yeast cells also undergo this pathway, since yeast is part of the bread dough rising process. Feedstocks such as corn are also fermented through ethanol fermentation, and the ethanol produced is used in gasoline.
In the context of the anaerobic respiration process, we start with two molecules of pyruvate that are produced via glycolysis. These pyruvates are broken down into acetaldehyde molecules, releasing two carbon dioxide molecules. The acetaldehyde molecules are then converted into ethanol, oxidizing NADH back to NAD+ for glycolysis to continue again and for the cycle to repeat. The enzymes pyruvate decarboxylase and alcohol dehydrogenase catalyze this reaction.
The reaction of this fermentation pathway is summarized by the following equation:
[math]\ce{ C6H12O6 -> 2C2H5OH + 2CO2 }[/math]
Lactic Acid Fermentation
Heterolactic and homolactic fermentation are two variations of lactic acid fermentation studied for this event, with the bifidium pathway being the third. None of these three are the lactic acid fermentation used in anaerobic respiration. Only the non-variated lactic acid fermentation is used in anaerobic respiration This type of fermentation is used for some dairy products, such as cheese and yogurt, and by mammal red blood cells and our muscle cells when there is a lack of oxygen, the reason for our cramps. This causes a build-up of lactic acid, used by our liver to produce pyruvic acid for metabolism processes. 2 molecules of pyruvate is converted into 2 molecules of lactate, and NADH is oxidized back to NAD+.
The following equation represents the reaction of lactic acid fermentation. The reaction is reversible, but the forward reaction is inhibited by acidic conditions.
[math]\ce{ 2 pyruvate + NADH <-> 2 lactate + NAD^+ }[/math]
Heterolactic Fermentation
A variation of lactic acid fermentation is the one mentioned in this section, heterolactic fermentation, also known as the heterofermentive process. Heterofermentative bacteria use this process, in which one molecule of glucose is converted into lactate, with the addition of the byproducts ethanol and carbon dioxide, along with one molecule of ATP through substrate-level phosphorylation with the use of the energy of the reaction.
The chemical reaction of this pathway is represented by the following equation.
[math]\ce{ glucose + ATP + Pi -> lactate + ethanol + CO2 + ATP }[/math]
Homolactic Fermentation
Similar to heterolactic fermentation, homolactic fermentation is another variation of lactic acid fermentation and is also known as the homofermentative process. Performed by homofermentative bacteria, glucose is converted to 2 molecules of lactate. Using the energy from this reaction, substrate-level phosphorylation is used to bind ADP to Pi to produce 2 molecules of ATP.
The chemical equation representing this reaction is shown below.
[math]\ce{ glucose + 2 ATP + 2 Pi -> 2 lactate + 2 ATP }[/math]
Food Preservation
The purpose of food preservation processes are to keep bacteria and molds from reproducing on or fermenting foodstuff before it is consumed. It prevents contamination with molds and pathogens and slows down enzymes that cause rancidity.
Low Temperature Preservation
Maintaining a low temperature in low-temperature preservation slows down the growth rates of microorganisms and any chemical or physical reactions (ex: oxidation). By converting water inside the food into ice, it is effectively lowering the aw (water activity) value of the food, preventing microbial growth.
Refrigeration
Refrigeration is the process of removing heat. This causes the life of foods to increase when refrigerated. It does not stop decay but slows it down. Refrigeration usually occurs at temperatures of 4 °C and under. Refrigeration is used to preserve foods such as fruits, vegetables, meat, dairy, etc. Microbial growth slows down starting from 50 °F. However, pathogenic bacteria cannot reproduce in temperatures less than 40 °F.
Freezing
Freezing is the removal of all heat from foods and slows down decay more extremely than refrigeration. It converts the water in food into ice. Freezing occurs at 0 °C and under. Freezing is used to preserve fish and meat.
Flash Freezing
Flash freezing is food being frozen within a few hours by subjecting them to cryogenic temperatures or through direct contact with liquid nitrogen at −196 °C (−320.8 °F). Flash freezing is also called lyophilization or cryodessication.
Thermal Processing
Thermal processing is the combination of heat and time to eliminate unwanted microorganisms or to destroy microorganisms that can cause spoilage or enzymes that can affect different characteristics of the food.
Canning
In canning, foods are canned once they reach a high temperature so that all microorganisms are gone & no more can enter again.
First, the food is cleaned. Then, it is canned and vacuumed airtight so that no oxygen-requiring organisms can survive (exhausting). Any remaining organisms are destroyed during the heating process. The food is then cooked, labeled, cased, and stored.
However, the downside of canning includes a loss of nutrients from heat processing and overheating of closer areas of the food from the heat source. Canning is used to preserve fruits, vegetables, soups, etc.
Sterilization
Unlike methods like pasteurization and disinfection, in sterilization, rather than killing all living organisms in the food, only certain ones are killed, namely the harmful and disease-spreading organisms. The food in this state is aseptic, or sterile, meaning free of disease-spreading microorganisms.
Sterilization can be accomplished through different methods, for example, heating. One scientist who made an impact on sterilization as we know it today is Nicholas Appert.
Microorganism Identification and Behaviors
Yeast
Yeast is a fungus that ferments in foods and drinks and is used to produce ethanol (alcohol) and carbon dioxide.
Beer
In beer, the strains Saccharomyces cerevisiae (for ale) and Saccharomyces pastorianus (for lager; used to be named Saccharomyces carlsbergensis) are used. The process of fermentation occurs in three stages: first, the yeast absorbs oxygen; then, it actively digests sugars and creates alcohol and carbon dioxide and forms krausen, the frothy layer that consists of proteins, by-products and yeast cells pushed up by carbon dioxide; finally, the yeast slows down and finishes digesting the final sugars and cleans up by-products while the krausen sinks.
Wine
In wine, strains from the genus Saccharomyces including the strains Saccharomyces cerevisiae (for general wines) and Saccharomyces bayanus (for fortified wine production like ports), as well as Brettanomyces (for complexity) are used. The yeast converts the sugars found in wine grapes into alcohol and carbon dioxide and depending on the wine, the duration of fermentation will change. For champagne, a second fermentation occurs to provide more carbonation by adding a small amount of sugared liquid.
Bread
In bread making, the strain Saccharomyces cerevisiae is used. The yeast feeds on sugars in the dough (glucose, levulose and sucrose) and sugars created from starch breaking down (maltose). This produces alcohol and carbon dioxide. Bread making also takes place in warm temperatures, which helps fermentation take place. The carbon dioxide produced creates tiny air pockets and expands, making the bread rise; the alcohol evaporates.
Non-alcoholic Beverages
Yeast is also used in non-alcoholic beverages and goes through the same method used to make beer, except fermentation is stopped sooner so only trace amounts of alcohol are produced. Some examples of these beverages: kefir/kumis (fermenting milk), kombucha (fermented sweet tea), mauby (fermenting sugar with wild yeasts) and root beer.
Water Activity
The water activity, or aw, of any substance, is defined as the ratio of the partial vapor pressure of water in that substance to the standard state partial water vapor pressure in this said substance, this standard state being the same partial vapor pressure of water at the same temperature of that of the substance. Water activity is measured from 0 to 1, with no units, with the aw of distilled water being 1. When the temperature increases, the aw generally increases, except in cases where table salt and/or sugar are used in foods, where it doesn't really change. In areas with higher aw levels, microorganisms thrive better. Between two areas of different aw values, the water moves from the substance with the higher aw to the substance with the lower aw until they reach an equilibrium, like the concept of thermal equilibrium, where warmer substances transfer their heat to cooler substances. It is measured by a dew point or capacitance hygrometer, or a resistive electrolytic.
Water activity may be represented by the following equation:
[math]\displaystyle{ a_w = \frac{p}{p_w} }[/math],
where p is the partial vapor pressure of water in that substance, and pw the partial vapor pressure of water at the same temperature as that of the substance.
Foods such as raw meat and jam jellies have a higher aw, while foods such as graham crackers or a granola bar have a lower aw. Notice that foods that are drier generally have a lower aw values, while foods with more moisture have a higher aw value since they contain more water and therefore have a partial vapor pressure of water (in the food) closer to that actually of water at the same temperature, and since microorganisms benefit from a moist environment, microorganisms are more likely to grow on foods with a higher aw, which is why it is important to preserve them.
Nutrition Labeling Regulations
The food label that appears on the front of a food product visible to consumers is called the PDP, which stands for the principal display label. It must show the name of the food product and the quantity or amount of the product.
A nutrition label is in the shape of a rectangle, no matter the shape of the container of the food product. Although Helvetica bold and regular is used a lot more commonly used than other fonts, other fonts are allowed to be used. However, the bolding, font sizes and formatting in terms of spacing must be specific on every nutrition label. This year, the nutrition label has changed quite a bit. The new nutrition labels increase the font size of the number of calories and remove the number of calories from fat, along with the serving size now being on the bottom of the number of servings per container, bolded and formatted differently as well. The percentage of daily values were also updated according to the new serving sizes of foods and beverages. Other changes include showing amounts of vitamins and minerals, replacing vitamins A and C with D and potassium since Americans don't get enough of these two, but do more of A and C, adding the amount of added sugar, and replacing the old footnote with a new one.
Chemical Feedstocks
A chemical feedstock is a raw material that is used to supply or fuel machines or industrial processes. For example, algae is used for hydrocarbon fuels and corn for ethanol. This happens because plants contain sugars, which can be fermented to make ethanol in the process of biochemical conversion.
Macromolecules
Macromolecules are very large molecules. There are conventionally four different biopolymers: lipids, carbohydrates, proteins, and nucleic acids. However, the last one (nucleic acid) does not apply to Food Science; therefore, the first three should be focused on. In each of these categories of macromolecules, some subcategories exist.
Lipids
Lipids are unique macromolecules, in that they are defined by their physical properties as opposed to their chemical structures. All lipids are united by the fact that they are soluble in non-polar solvents, meaning that they are hydrophobic and will not dissolve in water. This definition of lipids also means that they are an incredibly diverse group of biomolecules, having many subfamilies such as triacylglycerols (triglycerides), waxes, sterols, and phospholipids. The most important lipids for this event are triglycerides and sterols.
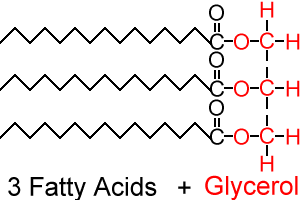
Triacylglycerols/Triglycerides
Triacylglycerols, also known as triglycerides, are molecules with a glycerol backbone and three fatty acid tails. Fatty acids are carboxylic acids with long carbon chains. The amount of double bonds present between the carbon molecules in these chains determines whether the molecule is saturated or unsaturated. The fatty acid tails in a triglyceride may be any combination of saturated or unsaturated, and do not have to be identical.
Fats are a good source of energy, providing 9 kcal of energy per gram of fat. The daily recommended amount of fat intake is limited to 65g. These contribute to a large amount of the obesity problem in the U.S.
Esters
Triacylglycerols are esters of glycerol, meaning that they have a C-O-C structure as part of the molecule. Because fatty acids are carboxylic acids, when they react with the glycerol backbone they form an ester. Anytime a carboxylic acid reacts with an alcohol, an ester will form. While the ester structure is only one part of the molecule, any molecule that contains an ester is classified as an ester.
Common esters include ethyl acetate and ethyl ethanoate.
Saturated Fatty Acids
Saturated fatty acids are one long chain of carbon atoms. They do not contain double bonds. Saturated fats are generally unhealthy since they clog arteries, increasing the risk of heart attack and stroke. Since the carbon atoms in a saturated fatty acid are packed closely together, saturated fats are usually solid at room temperature. They are generally found in animals.
Unsaturated Fatty Acids
Unsaturated fatty acids are also a long chain of carbon atoms, this time with one or more double bonds. Unsaturated fatty acids with one double bond are monounsaturated, and those with two or more double bonds are polyunsaturated. Unsaturated fats are generally healthier when not overeaten because they may help lower blood cholesterol level. Since double bonds exist, these fatty acids are much more wobbly and, therefore, are usually liquid at room temperature. Unsaturated fats are generally found in plants such as nuts and seeds. They are also found in fish as omega-3 unsaturated fatty acids.
Omega-3 Essential Fatty Acids
Omega-3 essential fatty acids are found in fish and plants. The name means there exists a double bond three carbon atoms from the non-ester end of the chain. Omega is the last letter in the Greek alphabet (lowercase omega is used). These fatty acids are "essential" because the body cannot produce them on its own, and they are vital for normal metabolism.
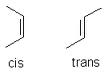
Trans Fats
Trans fats are not found in nature, although recent studies suggest that there may be small amounts. Trans fats are unsaturated fatty acids heated up then made so "dizzy" that it changes from cis to trans configuration.
Trans fats are unhealthy since they lower high-density lipoproteins (HDLs or "good" cholesterol) and raise low-density lipoproteins (LDLs or "bad" cholesterol). The recommended daily intake should be limited to 0g per day, but a little isn't going to cause problems.
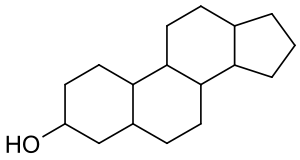
Sterols
The one sterol you'll want to know about is cholesterol. Cholesterol, like all sterols, come in this form:
Cholesterol comes in high-density lipoproteins (HDLs or "good" cholesterol) and low-density lipoproteins (LDLs or "bad" cholesterol. HDLs are made by your liver, while LDLs are generally consumed. Some types of foods, such as trans fats, are thought to raise LDL levels and lower HDL ones.
If a body has too much LDL, arteries will clog and result in a heart attack. This is cardiovascular disease, the leading cause of death in America.
Carbohydrates
Carbohydrates are, as suggested by the name, hydrates of carbon. They consist of carbon, oxygen, and hydrogen atoms. The formula for a carbohydrate can be expressed as [math]\displaystyle{ C_m(H_2 O)_n }[/math], where, most commonly, [math]\displaystyle{ m }[/math] and [math]\displaystyle{ n }[/math] are the same.
Carbohydrates include simple sugars (carbohydrates made of one or two molecules of sugar), monosaccharides (any of the class of sugars, e.g., glucose, that cannot be hydrolyzed to give a simpler sugar) and disaccharides (a sugar [i.e. carbohydrate] composed of two monosaccharides), and polysaccharides and oligosaccharides (a carbohydrate [e.g., starch, cellulose, or glycogen] whose molecules consist of several sugar molecules bonded together).
Simple Sugars
Simple sugars consist of single sugar units (monosaccharides) and disaccharides (which are made up of two monosaccharides). The names of sugars often end in the suffix -ose. Hexose sugars are 6 carbon monosaccharides while pentose sugars are 5 carbon monosaccharides. Some monosaccharides include:
- Glucose/Dextrose (hexose)
- Fructose/Levulose (hexose)
- Galactose (hexose)
- Mannose (hexose)
- Ribose (pentose)
- Xylose (pentose)
- Arabinose (pentose)
Some disaccharides include:
- Sucrose (glucose+fructose)
- Lactose (glucose+galactose)
- Maltose (only found as a byproduct of hydrolysis of starch; glucose+glucose)
- Trehalose (also found as a byproduct of hydrolysis of starch; glucose+glucose)
- Melibiose (formed when there is an alpha-1,6-glycosidic linkage between glucose+galactose)
Simple sugars are small, easy to break down, and, therefore, give energy quite soon after consumption. However, they run out quickly, leaving one tired. Think about crashing after a sugar high.
The "Sugars" on food labels consist of mono- and disaccharides. That's why you see sugar in milk; that's lactose, not added by the manufacturer.
Polysaccharides and Oligosaccharides
Polysaccharides are typically made up of 10 or more monosaccharides, while oligosaccharides are typically made up of three to 10 monosaccharides. Polysaccharides are chains of many monosaccharides, most commonly glucose.
Polysaccharides are divided into two main groups, storage polysaccharides and structure polysaccharides.
Storage Polysaccharides
Storage polysaccharides are our main source of energy. There are two main storage polysaccharides: glycogen and starch.
- Glycogen is the storage polysaccharide found in animals. Its purpose is to maintain blood glucose levels and serve as a readily mobilized energy source.
- Starch is the storage polysaccharide found in plants. We, humans, consume a lot of it from foods like pasta or potatoes.
Starch comes in two forms: amylose and amylopectin. Amylose is a straight chain of glucose molecules that coils up, while amylopectin is branched. Since there are more ends to be broken down in amylopectin, it is more quickly digested.
When looking at a food label, the Dietary Fiber and Sugars don't quite add up to the Total Carbohydrate; the remainder is starch.
Structure Polysaccharides
Structure polysaccharides are polysaccharides meant to give structure. Two common structure polysaccharides are cellulose and chitin.
- Cellulose is the structural polysaccharide found in plant cell walls. Cellulose is better known as (dietary) fiber. It is insoluble, which means it is indigestible by our bodies, so it cleans out our insides and comes out as feces.
- Chitin is the structural polysaccharide found in the cell walls in fungi, the exoskeletons of arthropods (think crustaceans and insects), the radula (teeth) of molluscs (think snails and clams), the beaks of cephalopods (think octopus and squids), and the scales of fish & lissamphibians (modern amphibians). Chitin is composed of long chains of N-acetylglucosamine, a derivative of glucose.
Proteins
Proteins are polymers of amino acids. Proteins are essential to human life because they carry out orders from the genes in cells.
Proteins can be converted to energy by the liver when there is a lack of carbohydrate or fat; therefore, they provide 4 kcal/g.
Protein Denaturation and Coagulation
Protein denaturation is the undoing of the natural structure by chemical or physical means. Denaturation doesn’t change the composition of the protein, only the structure. Protein denaturation can happen because of heat (140-180 degrees Fahrenheit/ 60-80 degrees Celsius), high acidity, air bubbles, or any combination of the three. Since denaturation changes the folds of the proteins, there are more open bonds, so they form new bonds. This creates a thickness or density. Coagulation is the process when these new bonds are formed.
Enzymes
Enzymes are a type of special proteins that catalyze chemical reactions. The names of enzymes often end in the suffix -ase. Examples are maltase (breaks down maltose), amylase (breaks down amylose and amylopectin), and lactase (breaks down lactose).
Some enzymes cause disease because some people do not contain them or possess distorted, non-functional forms. The most common disease is phenylketonuria (PKU), which is a lack of functional phenylalanine hydroxylase, an enzyme. When functional, phenylalanine hydroxylase is supposed to break down phenylalanine, an amino acid found in the artificial sugar aspartame.
Amino Acids
Amino acids are the building blocks of proteins. Amino acids consist of 10-40 atoms each, mainly carbon, hydrogen, sometimes sulfur, and at least one nitrogen in the amino group, -NH2. Proteins are formed by linking the amine nitrogen with a carbon atom on another amino acid, forming a peptide bond.
Complete and Incomplete Proteins
All amino acids are needed to live. There are 9 essential amino acids and 11 non-essential ones. The body does not make the essential ones, therefore they need to be consumed. The body makes the other 11 itself, therefore it is not essential to eat them. For a person with a normal diet, all 9 essential amino acids are normally found in most meats.
This poses a problem for vegetarians, who cannot eat proteins with all the essential amino acids. So they must eat complementary proteins, or two different food ingredients, when, eaten together, make complete protein sets that contain all the proteins that you need to eat. An example of a pair of complementary proteins is rice and beans.
Emulsification
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable). Emulsions are part of colloids. In an emulsion, one liquid (the dispersed phase) is dispersed in the other (the continuous phase). The word "emulsion" comes from the Latin word for "to milk", as milk is an emulsion of fat and water, among other components. Emulsions, being liquids, do not exhibit a static internal structure. The droplets dispersed in the liquid matrix (called the “dispersion medium”) are usually statistically distributed.
Hydrogenation
The chemical reaction between hydrogen (H2) and another compound or element, usually in the presence of a catalyst (nickel, palladium, or platinum). Reduces or saturates organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, generally an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons, hydrogenation of unsaturated fats produces saturated fats. Partial hydrogenation can produce trans fats.
Food Testing
At the competition, teams are expected to be prepared to perform certain experiments on the Approved List of Ingredients. Most tests will include instructions for performing the experiments, but it is good to be familiar with them beforehand
Sugar Concentration Testing
Hydrometers are needed to test the density of solutions, provided by the event supervisor, and made beforehand. If you do not bring a Hydrometer, the most amount of points you can get on the final score is 60%. There is between 1 and 3 solutions. Just bringing a hydrometer is worth 10% of the test. If there is one solution, that is worth 30% of the total score. If there are two solutions, then each one of them is worth 15% of the total score, for a total of 30%. If there are three solutions, then each of them is worth 10% of the total score, for a total of 30%. The answer should be between 1% and 10%(mass/volume). At regionals, you can get full credit if you are off by 1%. At states and nats, you get full credit if you are off by 0.5%(So mark your hydrometers for every 1/2 of a percent!). You should check with the tournament for the rules of invitationals.
Molecule Detection Tests
There are certain tests used to detect macromolecules and other molecules in food. Teams may have to perform some of these at the competition. The most common tests are Benedict's, Biuret's, Iodine, and the Brown Bag test.
Benedict's
Benedict's solution is also known as Fehling's solution. It tests for reducing sugars, or a sugar with a free aldehyde. The reaction between a reducing sugar and Benedict's is between the reducing sugar's aldehyde and the copper sulfate in Benedict's.
To use Benedict's:
- Put a small sample of the food into a test tube.
- Liquefy the food by adding enough water to make it a liquid, if the food is not already a liquid.
- Add 5-10 drops of Benedict's Solution.
- Carefully heat the test tubes in a hot water bath at 40-50 degrees Celsius (104-122 degrees Fahrenheit) for five minutes.
To deduce the results:
The liquid will turn green, yellow, or brick red depending on the amount of sugar present. Green is the least sugar, yellow is more, and red is the most. If negative, it will be blue.
Note that Benedict's will only work with reducing sugars, which are sugars with free aldehydes. (An aldehyde group is of the form R-CH=O where R is something organic). Here's a rule of thumb: all monosaccharides are reducing sugars but not all reducing sugars are monosaccharides. Lactose, for example, is reducing; however, sucrose is not. Make sure to know the reducing sugars, because trick questions often arise on tests on this subject.
Remember that Benedict's needs heating to work when answering test questions about it!
Biuret's
Biuret's Reagent is for detecting the presence of proteins. The active agent in Biuret's is also copper sulfate. The reaction is due to the formation of complex between the cupric ions in copper sulfate and the lone pair of electrons present on the nitrogen and oxygen atoms of peptide bonds of proteins.
To use Biuret's:
- Put a small sample of the food into a test tube.
- Liquefy the food by adding enough water to make it a liquid, if the food is not already a liquid.
- Add 2-5 drops of Biuret's Solution.
- Swirl gently to mix.
- Let sit for five minutes.
To deduce the results:
Biuret's will turn a pink/purple in the presence of proteins. If negative, the solution will have no color.
Iodine
Iodine solution, also Lugol's Iodine, is used to detect starch. It is a mix of the element iodine and potassium iodide. The reaction is the result of the formation of polyiodide chains from the reactive starch and iodine.
To use Iodine:
- Put a small sample of the food into a test tube.
- Liquefy the food by adding enough water to make it a liquid, if the food is not already a liquid.
- Add 2-5 drops of Iodine.
- Swirl gently to mix.
To deduce results:
The solution will turn dark blue, almost black, in the presence of starch. It will be brownish-yellow if negative.
Note that amylose (straight chain form of starch) will stain less than amylopectin (a branched form of starch).
Brown Bag
The brown bag is the easiest and least formal test. It tests for lipids (fats).
To perform this test, spread, rub or pour some of the food on a brown bag. Wipe away the excess, and hold the bag to the light. Foods containing more lipids will stain the bag more transparently than ones that have fewer lipids.
This test can also be done with plain paper, though the paper has to dry before it can be analyzed.
Density
Teams may have to find the density of certain baked foods made from ingredients on the Approved List of Ingredients such as bread. To do this, you will have to first cut it into a uniform cuboid (a 3-D rectangle). Next, use a ruler to measure the height, length, and breadth (AKA width) of the object. Record these measurements. Now, weigh the object. The event supervisor must provide a scale. Use the density formula to find the density of the food. Make sure to answer the units wanted. The most common units wanted is [math]\displaystyle{ \frac{g}{cm^3} }[/math].
The density formula is [math]\displaystyle{ \text{Density}=\frac{\text{weight}}{\text{height}\cdot\text{length}\cdot \text{breadth}} }[/math]
Competition Tips
- Study a lot. This event is one of the heaviest in terms of what teams need to know, therefore retention is crucial.
- Ask the event supervisor if tests can be unstapled and then later re-stapled. This strategy may help teams divide work to save valuable time.
- Get a lot of rest before the competition! Self-explanatory. Also, eat a big breakfast. Don't stay up too late the day before competition studying
- Make sure to be able to recognize the structures of basic molecules very quickly (mono and disaccharides, triglycerides, etc)
- Don't stress!