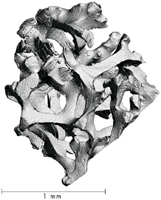Materials Science
This page is incomplete. |
| Materials Science | ||||||
|---|---|---|---|---|---|---|
| Type | Chemistry | |||||
| Category | Lab | |||||
| Event Information | ||||||
| Latest Appearance | 2017 | |||||
| Forum Threads | ||||||
| ||||||
| Question Marathon Threads | ||||||
| ||||||
Materials Science is a Division C event which tests knowledge of the properties and characteristics of metals, ceramics, polymers and composite materials, with a focus on material characterization techniques, intermolecular forces, and surface chemistry.
In 2013, 2014, and 2017 the event focused on general materials science. For the 2018 season, the event will focus on polymers and plastics.
Event Overview
Rules Review
For 2018, each team can bring 2 double-sided pages of information into the event. Students will be tested on the field of Materials Science and topics are limited to those listed below, though the scope of each topic is nearly unlimited. There is a lab section unless otherwise noted.
Event supervisors will provide any needed materials and constants. Students must provide all safety equipment for the event. This includes Category C goggles and a lab coat/chemical apron, while gloves are optional. Students must wear closed-toed shoes and full length pants or skirts in order to compete.
Tips and Tricks
Materials Science covers a vast amount of knowledge. The extent of each topic is nearly unlimited, such that teams will need to know each topic well without relying on cheat sheets. Graphs are especially important, but only so long as they are useful - too many basic graphs will take up valuable space. Also, cheat sheets can contain large tables of information that would otherwise be impossible to memorize, such as atomic packing for different elements and compounds, melting and boiling points, and more. Many teams have found an optimal layout of 0.5" margins, 3 columns, and Times New Roman font. The font size will depend largely on the amount of information contained, but teams should be cautious to still keep their cheat sheets readable. The best competitors will know their cheat sheets extraordinarily well, such that they can find small bits of information quickly. Less competitive teams will either rely on their cheat sheets for basic information or rely on the find function during practice and become lost in actual tests. Lastly, screen protectors are incredibly useful for protecting cheat sheets from any damage or spills in the lab section of the event.
Physical Characteristics
Metals
Metals use metallic bonds to form their structures. Metallic bonds constitute positively charged metal ions in a "sea" of delocalized electrons.In metallic bonds, electrons are free to move around the structure of metal ions, meaning that electrons belonging to molecules are not attached to a specific atom in that molecule. This allows metals to have unique properties.
- Low Hardness - Metallic bonds give the metal ions freedom to move around. This means that the metal can change shape without breaking any bonds, due to the free nature of metallic bonding.
- High Elastic Modulus
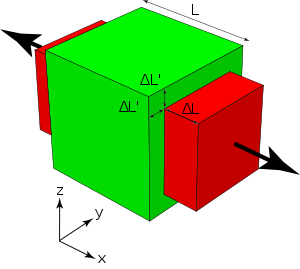
- Low Thermal Expansion - When placed in a high-heat environment, metals will typically expand very little. The exact change can be determined by the equation [math]\displaystyle{ \Delta L = \alpha T L_i }[/math], where [math]\displaystyle{ \alpha }[/math] is the coefficient of linear thermal expansion, [math]\displaystyle{ T }[/math] is the temperature of the surroundings (in Celsius), and [math]\displaystyle{ L_i }[/math] is the initial length of the bar.
- High Ductility - The freedom of movement of electrons in metallic bonding also allows for free movement of ions. This means that the metal can be pulled into wires without breaking any bonds, meaning it does not take large amounts of energy to do.
- Low Corrosion Resistance - All the free electrons in metallic bonds tempt outside elements, usually oxygen, to steal pieces of the metal. Rust is a well known byproduct of corrosion of iron. It is done through the chemical process of oxidation. It usually forms either oxides or salts of the metal.
- High Electrical Conductivity - The freedom of electrons in metallic bonds allow them to travel across molecules without difficulty, which allows them to carry a charge across the metal.
- High Density - Many metals assume a BCC, FCC, or HCP arrangement in the crystalline state. See Common Atom Packing Materials Science#Common Atomic Packing for more information.
- High Thermal Conductivity - Metals have high thermal conductivity for the same reason they have high electrical conductivity. Not only do the free electrons have the ability to transfer charge, they also make it easy to transfer heat along the metal.
- High Boiling Point - The strength of metallic bonds require a lot of energy to break. This means that most metals have high boiling points. Except for a few notable cases, all metals have high boiling points. Even Gallium, which melts in the hand, has a boiling point close to that of copper due to the strength of the bonds.
- Magnetic Properties
- Diamagnetism: Diamagnetism is present in all materials. An applied magnetic field induces another magnetic field in the opposite direction, providing a repulsive force. Diamagnetic materials could be considered non-magnetic, as diamagnetism is negligible compared to other, stronger forms of magnetism.
- Paramagnetism: Paramagnetism exists due to unpaired electrons in some elements and compounds, which generate a magnetic dipole due to their spin. Paramagnets do not retain magnetization unless they are in the presence of an applied magnetic field.
- Ferromagnetism: Ferromagnetism also exists due to unpaired electrons, but works in a different manner. Magnetic moments align themselves parallel to each other and to an applied magnetic field in order to maintain a lowered state. These magnetic moments align even in the absence of an applied magnetic field. Every ferromagnetic material has a Curie temperature, above which it loses any ferromagnetic properties.
Ceramics
Ceramics are relatively stiff and strong, hard, brittle, susceptible to fracture, insulators of heat and electricity, and are very resistant to high temperatures and harsh conditions. Some ceramics (glass ceramics) can be transparent or translucent, while others (clay ceramics) are opaque. Some oxide ceramics also exhibit magnetic behavior.
Polymers
For more information about polymers, please see Materials Science/Polymers.
Mechanical properties: Polymers are low density, but not as stiff or strong as metals or ceramics. They are extremely ductile and pliable, chemically inert in a multitude of environments, nonmagnetic (diamagnetic), and usually are not conductive (polymers with a backbone of alternative double and single bonded carbon can conduct electricity through the movements of free electron pairs). Polymers soften or decompose at modest temperatures.
Degree of polymerization: The degree of polymerization, or DP, is defined as the number of monomer units in a macromolecule or polymer molecule. A homopolymer contains only one type of monomer unit, and the number-average degree of polymerization is given by [math]\displaystyle{ M_n/M_0 }[/math] where M(n) is the number-average molecular weight and M(0) is the molecular weight of the monomer unit.
Composites
The properties of composites are entirely dependent on their constituent parts and are usually enhanced by fiber lengths. Strength is dependent on fiber length, where smaller fibers are larger. Ductility, meanwhile, can be lowered by using matrices instead of fibers. Ceramic composites are resistant to degradation, but are brittle; metal matrices are used at high temperatures. Construction also matters. Carbon-carbon composites, which reinforce graphite with fibers, have high tensile strength, resistance to creep, fracture toughness, strength, and thermal conductivity; laminar composites, meanwhile, have high strength in the 2D plane only; sandwich panel cores have low elasticity, light weight, and high shear strength, while their sheets resist tension and compression.
Manufacturing Techniques and Natural Occurrences
Metals
Natural Occurrences
Metals are said to be in their native state if they are found in their elementary form. Generally, less active metals like gold, silver, copper, and platinum can be found in their native state.
Metals are said to be in their combined state if they are found in nature in the form of compounds. Generally, reactive metals occur in their combined state. Combined state metals are found in the crust of the earth as oxides, carbonates, sulfides, silicates, phosphates, etc.; these are known as minerals. Minerals extracted from the crust of the earth are not pure, but instead associated with many earthly, rocky, and siliceous impurities. These impurities associated with minerals are collectively known as the gangue or matrix, which is commercially valueless. Not all minerals can be used, depending on economic factors or the extraction process. Minerals from which metals can be conveniently and economically extracted are known as ore. For example, aluminum can be found naturally in two minerals, both bauxite and China clay. However, extraction of aluminum is much cheaper and easier from bauxite than from China clay, so bauxite is considered an ore of aluminum while China clay is not. Similarly, copper can be found as copper glance, cuprite, malachite, and copper pyrite, but only copper pyrite is considered to be an ore of copper. All ores are minerals, but not all minerals are considered to be ores.
Mining
Metals are often extracted from Earth by means of mining ores that are rich sources of requisite elements, such as bauxite. Ore is located by prospecting techniques, followed by exploration and examination of deposits. Mineral sources are generally divided into surface mines, which are mined by excavation using heavy equipment, and subsurface mines. Once Ore is mined, metals must be extracted, usually by chemical or electrolytic reduction. Pyrometallurgy uses high temperatures to convert ore into raw metals, while hydrometallurgy employs aqueous chemistry for the same purpose.methods used depend on metal and their contaminants. When a metal ore is an ionic compound of that metal and a nonmetal, the ore must usually be smelted — heated with a reducing agent — to extract the pure metal. Many common metals, such as iron, are smelted using carbon as a reducing agent. Some metals, such as aluminum and sodium, have no commercially practical reducing agent and are extracted using electrolysis instead. Sulfide ores are not reduced directly to metal but are roasted in air to convert them to oxides.
Forming Techniques
- Hot Working: Hot working is when a metal is deformed at a temperature higher than its temp of recrystallization.
- Forging: Forging is mechanically working or deforming a single piece of a normally hot metal; this may be accomplished by the application of successive blows or by continuous squeezing. Forgings are classified as either closed or open die. For the closed die, a force is brought to bear on two or more die halves having the finished shape such that the metal is deformed in the cavity between them. For the open die, two dies having simple geometric shapes (e.g., parallel flat, semicircular) are employed, normally on large workpieces. Forged articles have outstanding grain structures and the best combination of mechanical properties. Wrenches, and automotive crankshafts and piston connecting rods are typical articles formed using this technique.
- Rolling: Rolling, the most widely used deformation process, consists of passing a piece of metal between two rolls; a reduction in thickness results from compressive stresses exerted by the rolls. Cold rolling may be used in the production of sheet, strip, and foil with a high-quality surface finish. Circular shapes, as well as I-beams and railroad rails, are fabricated using grooved rolls.
- Extrusion: For extrusion, a bar of metal is forced through a die orifice by a compressive force that is applied to a ram; the extruded piece that emerges has the desired shape and a reduced cross-sectional area. Extrusion products include rods and tubing that have rather complicated cross-sectional geometries; seamless tubing may also be extruded.
- Drawing: Drawing is the pulling of a metal piece through a die having a tapered bore by means of a tensile force that is applied on the exit side. A reduction in cross section results, with a corresponding increase in length. The total drawing operation may consist of a number of dies in a series sequence. Rod, wire, and tubing products are commonly fabricated in this way.
Casting Techniques
Casting is when a heated metal is poured into a mold in order to achieve a desired shape.
- Sand Casting: With sand casting, probably the most common method, ordinary sand is used as the mold material. A two-piece mold is formed by packing sand around a pattern that has the shape of the intended casting. Furthermore, a gating system is usually incorporated into the mold to expedite the flow of molten metal into the cavity and to minimize internal casting defects. Sand-cast parts include automotive cylinder blocks, fire hydrants, and large pipe fittings.
- Die Casting: In die casting, the liquid metal is forced into a mold under pressure and at a relatively high velocity and allowed to solidify with the pressure maintained. A two piece permanent steel mold or die is employed; when clamped together, the two pieces form the desired shape. When complete solidification has been achieved, the die pieces are opened and the cast piece is ejected. Rapid casting rates are possible, making this an inexpensive method; furthermore, a single set of dies may be used for thousands of castings. However, this technique lends itself only to relatively small pieces and to alloys of zinc, aluminum, and magnesium, which have low melting temperatures.
- Investment Casting: For investment (sometimes called lost wax) casting, the pattern is made from a wax or plastic that has a low melting temperature. Around the pattern is poured a fluid slurry, which sets up to form a solid mold or investment; plaster of Paris is usually used. The mold is then heated, such that the pattern melts and is burned out, leaving behind a mold cavity having the desired shape.This technique is employed when high dimensional accuracy, reproduction of fine detail, and an excellent finish are required—for example, in jewelry and dental crowns and inlays. Also, blades for gas turbines and jet engine impellers are investment cast.
- Lost Foam Casting: A variation of investment casting is lost foam (or expendable pattern) casting. Here the expendable pattern is a foam that can be formed by compressing polystyrene beads into the desired shape and then bonding them together by heating. Alternatively, pattern shapes can be cut from sheets and assembled with glue. Sand is then packed around the pattern to form the mold. As the molten metal is poured into the mold, it replaces the pattern which vaporizes. The compacted sand remains in place, and, upon solidification, the metal assumes the shape of the mold. With lost foam casting, complex geometries and tight tolerances are possible. Furthermore, in comparison to sand casting, the lost foam is a simpler, quicker, and less expensive process, and there are fewer environmental wastes. Metal alloys that most commonly use this technique are cast irons and aluminum alloys; furthermore, applications include automobile engine blocks, cylinder heads, crankshafts, marine engine blocks, and electric motor frames.
- Continuous Casting: At the conclusion of extraction processes, many molten metals are solidified by casting into large ingot molds.The ingots are normally subjected to a primary hot-rolling operation, the product of which is a flat sheet or slab; these are more convenient shapes as starting points for subsequent secondary metal-forming operations (i.e., forging, extrusion, drawing). These casting and rolling steps may be combined by a continuous casting (sometimes also termed “strand casting”) process. Using this technique, the refined and molten metal is cast directly into a continuous strand that may have either a rectangular or circular cross section; solidification occurs in a water-cooled die having the desired cross-sectional geometry. The chemical composition and mechanical properties are more uniform throughout the cross sections for continuous castings than for ingot-cast products. Furthermore, continuous casting is highly automated and more efficient.
Miscellaneous Techniques
Power Metallurgy:
Yet another fabrication technique involves the compaction of powdered metal, followed by a heat treatment to produce a more dense piece. The process is appropriately called powder metallurgy, frequently designated as P/M. Powder metallurgy makes it possible to produce a virtually nonporous piece having properties almost equivalent to the fully dense parent material. Diffusional processes during the heat treatment are central to the development of these properties. This method is especially suitable for metals having low ductilities since only small plastic deformation of the powder particles need to occur. Metals having high melting temperatures are difficult to melt and cast, and fabrication is expedited using P/M.
Ceramics
This section is incomplete. |
Polymers
This section is incomplete. |
Polymer Additives
Composites
This section is incomplete. |
Chemical Tests
This section is incomplete. |
Chemical Composition of Materials
Surface Tension
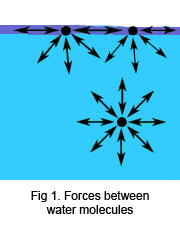
The contractive tendency of the surface of a liquid that allows it to resist an external force. It is caused by the cohesion of liquid molecules. This cohesion draws atoms towards each other. At the surface however, there are no atoms pulling down. This means all the forces are pointing up, which cause objects to float.
Surface tension is represented by [math]\displaystyle{ \gamma }[/math] and has the units of force along a unit length, where the force is parallel with the surface but perpendicular with the line (meaning the force is directed towards the liquid). For example, water has a surface tension of around 72 dynes/cm (at 25° C). The SI unit is newtons per meter, the cgs unit is dynes per cm. One dyn/cm corresponds to 0.001 N/m.
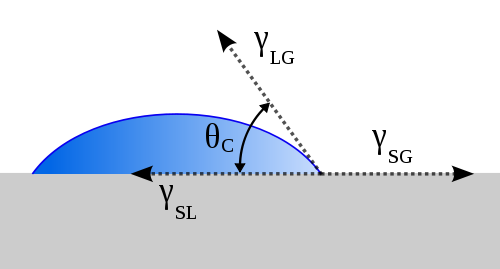
Contact Angle
The angle, conventionally measured through the liquid, where a liquid/vapor interface meets a solid surface, and is used to determine the "wettability" of a substance (such as a non-stick pan or waterproof fabric). The angle is the measure between a line tangent to the point of contact between the liquid and solid, and the surface of a material, [math]\displaystyle{ \theta }[/math] in the following picture.
The static sessile drop method uses a profile picture of the drop to determine this angle. This type of picture will be similar to the one above.
Contact angles are sensitive to contamination, with values reproducible to better than a few degrees only obtainable under laboratory conditions. For bare metal or ceramic surfaces, a contact angle of 0° is common. In general, surfaces with a contact angle of greater than 90° are considered hydrophobic, under 90° considered hydrophilic.
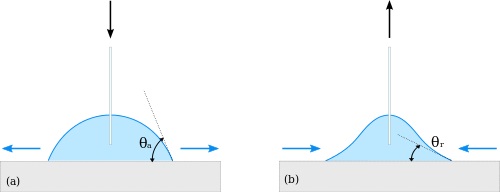
There are actually two types of contact angles. If a small amount of liquid is added, the contact angle will increase without changing the radius of the drop. This contact angle is called the advancing contact angle, [math]\displaystyle{ \theta_A }[/math]. If a small amount of liquid is removed, the contact angle will decrease. This is called the receding contact angle, [math]\displaystyle{ \theta_R }[/math]. This is the dynamic sessile drop method.
The difference between the two angles, [math]\displaystyle{ \theta _A - \theta_R }[/math] is the contact angle hysteresis
Crystal Structures
Atomic Packing
| Type | Description | Image | |
|---|---|---|---|
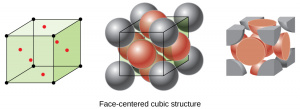
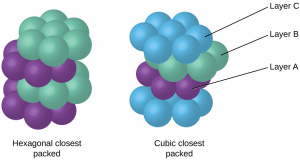
| Face Centered Cubic | Also known as cubic packed.
Atoms are located at each of the 8 corners as well as in the centers of each of the 6 faces. Follows an ABCABC close packing pattern - there are 3 repeating layers, where the atoms of the third layer are located above holes in the first and second layers. Densest of the cubic packing arrangements, with an atomic packing factor of 0.74. Each unit cell contains 4 atoms and has a side length of [math]\displaystyle{ A = 4R/\sqrt{2} }[/math]. Each atom in the matrix has a coordination number of 12. |
||
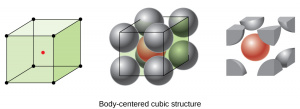
| Body Centered Cubic | Atoms are located at each of the 8 corners as well as in the center of the cubic cell.
Less dense than FCC, with an atomic packing factor of 0.68. Each unit cell contains 2 atoms and has a side length of [math]\displaystyle{ A = 4R/\sqrt{3} }[/math]. Each atom in the matrix has a coordination number of 8. |
||
| Hexagonal Close Packing | Another close-packed arrangement.
Composed of two hexagons of 6 atoms each, an additional atom in the center of each hexagon, and a triangle of atoms in between the two hexagons. Differs from FCC in that HCP follows an ABAB packing pattern - there are only 2 repeating layers, where the atoms of the third layer are located above the atoms of the first layer, not above gaps. Has an atomic packing factor of 0.74, the maximum possible. Each unit cell contains 6 atoms and has two parameters, A (side length) and B (height). Each atom in the matrix has a coordination number of 12. |
||
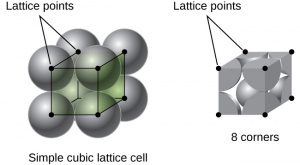
| Simple Cubic | Simple cubic is a very basic arrangement, only containing atoms at each corner of the unit cell.
SC is the least dense, with an atomic packing factor of 0.52. Each unit cell contains 1 atom and has a side length of [math]\displaystyle{ A = 2R }[/math] Each atom in the SC matrix has a coordination number of 6. |
Atomic Packing Factor (APF)
The atomic packing factor describes the amount of space occupied by atoms. For example, in the simple cubic packing, each cell has side length 2R and contains 1 atom of radius R.
The volume occupied by the atom is:
While the total volume is:
The fraction of space occupied by atoms is:
This exact amount can be seen listed above. This equation will work for any of the other crystal structures assuming the correct side lengths are used.
Crystal Types
Bond Types
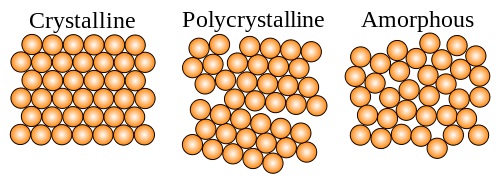
Crystallinity
Crystallinity refers to the degree of structural order in a solid. There are three degrees of crystallinity. The three types are shown in the following picture, and explained below.
- Crystalline - A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an ordered pattern extending in all three spatial dimensions. Large crystals are usually identifiable by their macroscopic geometrical shape, consisting of flat faces with specific, characteristic orientations. Crystal structures are repeats of a Unit Cell.
- Semi-Crystalline - A structure that has both Crystalline and Amorphuos properties. they are also known as polycrystalline structures. These structures have true crystal portions with mixed size and orientation. Semi-crystalline solids are also heavily bonded but lack the rigidity and constant structure of crystalline solids. Almost all metals, and many ceramics, are polycrystalline.
- Amorphous - A structure with little to no crystal properties. Amorphous solids still possess short-range order but have significantly less chain linkage. Common types of amorphous solids include gels, thin films, and glass.
Polymers are usually semi-crystalline to some degree, with some crystalline regions with rigid chain linkage and some amorphous regions. Metals follow a relatively crystalline structure. Finally, ceramics vary in crystallinity, from highly crystalline, vitrified fired ceramics to amorphous glasses.
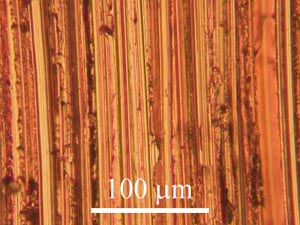
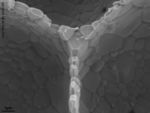
Visual Characterization
This section is incomplete. |
Physical Tests
Young's Modulus
Young's modulus describes a materials resistance to linear strain, like pulling a wire or placing a weight on a column.
[math]\displaystyle{ E=\frac{\sigma}{\epsilon}=\frac{FL_0}{A_0 \Delta L} }[/math]
- [math]\displaystyle{ E }[/math] is the Young's modulus (modulus of elasticity)
- [math]\displaystyle{ F }[/math] is the force exerted on an object under tension (a compressive force is represented by a negative value)
- [math]\displaystyle{ A_0 }[/math] is the original cross-sectional area through which the force is applied;
- [math]\displaystyle{ \Delta L }[/math] is the amount by which the length of the object changes;
- [math]\displaystyle{ L_0 }[/math] is the original length of the object.
The force may be found using Hooke's Law, [math]\displaystyle{ F=-kx }[/math] where
- [math]\displaystyle{ x }[/math] is the displacement of the spring's end from its equilibrium position.
- [math]\displaystyle{ k }[/math] is a constant called the rate or spring constant.
- [math]\displaystyle{ F }[/math] is the restoring force exerted by the spring on that end.
Yield Strength
The Yield Strength of a material is the force at which the material begins to deform plastically, or the force at which changes are not fully reversible.It is determined through a Stress-Strain curve, such as the one below.
- True elastic limit
- Proportionality limit
- Elastic limit(yield strength)
- Offset yield strength
For some materials (e.g., metals and plastics), change from elastic to plastic cannot be easily identified. Therefore, an offset method to determine the yield strength of the material tested is used. An offset is specified as a % of strain (for metals, usually 0.2%, for plastics, usually a value of 2%). The stress that is determined from the intersection point when the line of the linear elastic region (with slope equal to the Young's Modulus) is drawn from the offset becomes the Yield Strength by the offset method. For example, in the above image, an ofset of 0.2% is used for the yield strength.
Surface Area/Volume Ratio
As simple as it sounds, the ratio can be found by dividing the Surface area of an object by its volume, both of which can be determined by simple geometry. Typically, as a shape gets bigger, its surface area to volume ratio tends to decrease.
Creep Rate
In materials science, creep (sometimes called cold flow) tendency of a solid material to move slowly or deform permanently under influence of mechanical stresses. It can occur as a result of long-term exposure to high levels of stress that are still below yield strength of material. Creep is more severe in materials that are subjected to heat for long periods, and generally increases as they near their melting point.
The rate of deformation is a function of material properties, exposure time, exposure temperature and applied structural load. Depending on magnitude of applied stress and its duration,deformation may become so large that a component can no longer perform its function — for example creep of a turbine blade will cause blade to contact casing, resulting in failure of blade. Creep is usually of concern to engineers and metallurgists when evaluating components that operate under high stresses or high temperatures. Creep is a deformation mechanism that may or may not constitute a failure mode. For example, moderate creep in concrete is sometimes welcomed because it relieves tensile stresses that might otherwise lead to cracking.
Unlike brittle fracture, creep deformation does not occur suddenly upon application of stress. Instead, strain accumulates as a result of long-term stress. Therefore, creep is a "time-dependent" deformation.
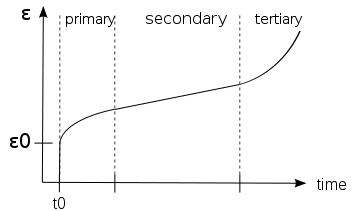
Stages of Creep
In the initial stage, or primary creep, strain rate is relatively high, but slows with increasing time. This is due to work hardening. Strain rate eventually reaches a minimum and becomes near constant. This is due to balance between work hardening and annealing (thermal softening). This stage is known as secondary or steady-state creep. This stage is most understood. Characterized "creep strain rate" typically refers to rate in this secondary stage. Stress dependence of this rate depends on creep mechanism. In tertiary creep, strain rate exponentially increases with stress because of necking phenomena or internal voiding decreased effective area of specimen. Fracture always occurs at tertiary stage.
Mechanisms of Creep
The mechanism of creep depends on temperature and stress. Various mechanisms are:
- Bulk diffusion (Nabarro-Herring creep)
- Climb — here strain is actually accomplished by climb
- Climb-assisted glide — here climb is an enabling mechanism, allowing dislocations to get around obstacles
- Grain boundary diffusion (Coble creep)
- Thermally activated glide — e.g., via cross-slip
Viscosity
Viscosity is "the resistance to flow."
Viscosity is caused by friction between molecules that move at different velocities. When in a tube, for example, the adhesion to the walls causes the outer material to move slower, while the material in the center moves faster. This means that some stress is required for the liquid to move. The more viscous the material, the more stress is needed.
A simple test of viscosity is to simply fill a graduated cylinder with the test liquid, and time how long a steel ball(or some other heavy object) takes to reach the bottom. While not giving an "accurate" measure of viscosity, or a number in terms of viscosity's actual units(which is in Pascals times seconds, [math]\displaystyle{ Pa }[/math]·[math]\displaystyle{ s }[/math]), it does provide a good comparison between liquids, and is a test that could be found in the lab portion of the event.
Fracture Toughness
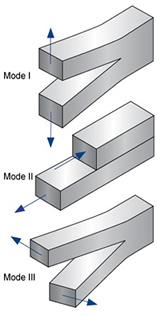
Fracture toughness is the ability of a material containing a crack to resist fracture.
The linear-elastic fracture toughness of a material is determined from the stress intensity factor ([math]\displaystyle{ K }[/math]). It is measured in [math]\displaystyle{ Pa }[/math]·[math]\displaystyle{ \sqrt{m} }[/math].
There are three types of tests for fracture toughness, diagrams of which can be found below.
Mode I is the most common, and the following equation can be used to determine the stress intensity factor.
[math]\displaystyle{ K_I }[/math] can be found by using the following:
[math]\displaystyle{ K_I=\sigma\sqrt{\pi a \beta} }[/math]
- Where [math]\displaystyle{ K_I }[/math] is the fracture toughness,
- [math]\displaystyle{ \sigma }[/math] is the applied stress (in [math]\displaystyle{ Pa }[/math])
- [math]\displaystyle{ a }[/math] is the crack length (in meters)
- [math]\displaystyle{ \beta }[/math] is a crack length and component geometry factor that is different for each specimen and is dimensionless.
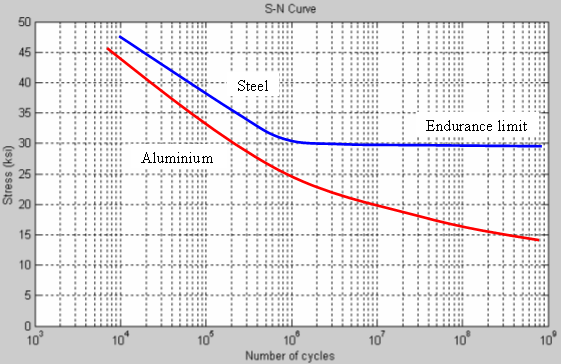
Fatigue Limit
Fatigue limit, endurance limit, and fatigue strength are all expressions used to describe a property of materials:amplitude (or range) of cyclic stress that can be applied to material without causing fatigue failure. Ferrous alloys and titanium alloys have a distinct limit, an amplitude below which there appears to be no number of cycles that will cause failure. Other structural metals such as aluminium and copper, do not have a distinct limit and will eventually fail even from small stress amplitudes. In these cases, a number of cycles (usually 107) is chosen to represent fatigue life of material.
The following is an example of a Fatigue Limit test.
The X-axis is a log scale of the number of cycles(of the stress). The Y axis is the amount of stress. The lines show the number of cycles at a given stress for failure to occur. For example, at about 45 ksi, the aluminium fails after 10000 cycles.
At the endurance limit, the steel does not break under even large number of cycles at low stress, while the aluminium does not show this property.
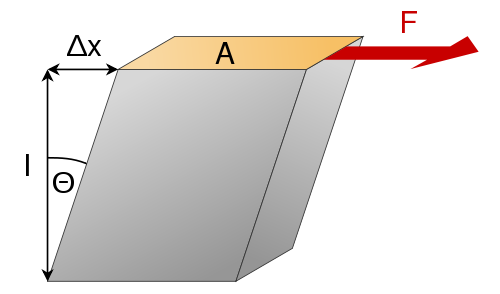
Shear Modulus
The shear modulus is equal to the shear stress over the shear strain, and is a measure of rigidity. It describes a materials resistance to shearing strains, like that of dull scissors.
[math]\displaystyle{ G=\frac{{F}{L}}{A \Delta x} }[/math]
- Where [math]\displaystyle{ G }[/math] is the Shear Modulus,
- [math]\displaystyle{ F }[/math] is the force,
- [math]\displaystyle{ L }[/math] is the original length of the object,
- [math]\displaystyle{ A }[/math] is the area on which the force acts
- [math]\displaystyle{ \Delta x }[/math] is the transverse displacement.
This diagram shows where those factors are.
Poisson's Ratio
Poisson's Ratio is the measure of how much a material expands(or contracts) when stretched or squeezed. Most objects have a positive Poisson's ratio, meaning they expand when squeezed and contract when stretched. However, some materials do exist with a negative ratio. The value of the ratio is [math]\displaystyle{ -1.0\lt \nu \lt 0.5 }[/math] due to the requirement for the Young's, shear, and bulk modulus to be positive.
[math]\displaystyle{ \nu=-\frac{d\epsilon_y }{d\epsilon_x } }[/math]
- Where [math]\displaystyle{ \nu }[/math] is Poisson's ratio,
- [math]\displaystyle{ d\epsilon_y }[/math] is transverse or lateral strain (negative for (stretching), positive for compression)
- [math]\displaystyle{ d\epsilon_x }[/math] is axial strain (positive for tension, negative for compression).
Bulk Modulus
The bulk modulus (K or B) of a substance is a measure of how compressible that substance is. It is defined as the ratio of infinitesimal pressure increase resulting relative decrease of volume. In addition, The bulk modulus K>0 can be formally defined by equation
[math]\displaystyle{ K = -V \frac{dP}{dV} }[/math]
where P is pressure, V is volume, and dP/dV denotes derivative of pressure with respect to volume. It is also equal to [math]\displaystyle{ K=\rho \frac{dP}{d\rho} }[/math] where ρ is density and dP/dρ denotes derivative of pressure with respect to density (i.e. pressure rate of change with volume). The inverse of bulk modulus gives a substance's compressibility. Other moduli describe the material's response (strain) to other kinds of stress: shear modulus describes the response to shear, and Young's modulus describes the response to linear stress. For a fluid, only the bulk modulus is meaningful. For an anisotropic solid such as wood or paper, these three moduli do not contain enough information to describe its behavior, and one must useful generalized Hooke's law.
Thermodynamic Relation: Strictly speaking, bulk modulus is a thermodynamic quantity, and in order to specify a bulk modulus it is necessary to specify how temperature varies during compression: constant temperature (isothermal KT) constant-entropy (isentropic KS) and other variations are possible. Such distinctions are especially relevant for gases.
Measurement: It is possible to measure bulk modulus using powder diffraction under applied pressure. It is a property of a fluid which shows its ability to change its volume under its pressure.
Notes
s1dav1s' Materials Science Notes
External Links
| Division B: Flying Bird · MagLev · Wright Stuff Capacitor | Division C: Flying Bird · Materials Science · Robot Arm |