Density Lab B
-
azboy1910
- Exalted Member

- Posts: 146
- Joined: Sat Nov 03, 2018 2:19 pm
- Division: B
- Pronouns: He/Him/His
- Has thanked: 117 times
- Been thanked: 62 times
Density Lab B
Let's get this marathon started!
1. A floating object displaces 40 g of liquid when placed in a tub full of it. What is its weight, in N?
2. What is the force required to hold a floating ball with a diameter of 5 mm and a mass of 25 g underwater (freshwater)?
3. 50 mL of water is poured into a cup. A person drinks 35 mL of it, and then places an object with a mass of 2 g in it. The object is completely submerged, and the water level has risen to 16.5 mL. What is the object's density in g/cc?
1. A floating object displaces 40 g of liquid when placed in a tub full of it. What is its weight, in N?
2. What is the force required to hold a floating ball with a diameter of 5 mm and a mass of 25 g underwater (freshwater)?
3. 50 mL of water is poured into a cup. A person drinks 35 mL of it, and then places an object with a mass of 2 g in it. The object is completely submerged, and the water level has risen to 16.5 mL. What is the object's density in g/cc?
-
YellowMamba
- Member

- Posts: 15
- Joined: Wed Sep 30, 2020 12:57 pm
- Division: B
- State: KS
- Has thanked: 0
- Been thanked: 0
Re: Density Lab B
1. Less than 392.4 N. 3. Assuming cc is cubic centimeters (correct me if I am wrong), 1.333 g/cc
-
azboy1910

- Exalted Member

- Posts: 146
- Joined: Sat Nov 03, 2018 2:19 pm
- Division: B
- Pronouns: He/Him/His
- Has thanked: 117 times
- Been thanked: 62 times
Re: Density Lab B
YellowMamba wrote: ↑Thu Oct 01, 2020 11:02 amOn 2 are you holding the ball in the air?1. Less than 392.4 N. 3. Assuming cc is cubic centimeters (correct me if I am wrong), 1.333 g/cc
1. The correct answer is 0.392 N, and when rounded to significant figures is 0.4 N. 40 g / 1000 = .04 kg .04 kg x 9.81 m/s^2 = 0.392 N 2. Since the ball already has a density greater than freshwater, there is no force required to hold the ball underwater. Hence, the answer here is 0 N. 3. Correct. If this was rounded to significant figures, the answer would be 1 g/cc.
Last edited by azboy1910 on Thu Oct 01, 2020 8:28 pm, edited 1 time in total.
-
YellowMamba
- Member

- Posts: 15
- Joined: Wed Sep 30, 2020 12:57 pm
- Division: B
- State: KS
- Has thanked: 0
- Been thanked: 0
Re: Density Lab B
A container of gas has a volume of 8 liters at one atmosphere of pressure and 40˚ Celsius. The temperature is then lowered to -40 Celsius, and the container is compacted to 4.2 liters. What is the final pressure in kPa? (6 sig figs)
-
azboy1910

- Exalted Member

- Posts: 146
- Joined: Sat Nov 03, 2018 2:19 pm
- Division: B
- Pronouns: He/Him/His
- Has thanked: 117 times
- Been thanked: 62 times
Re: Density Lab B
Since no one has answered yet,
Answer: 143.965 kPa Where 1 atm = 101.325 kPa, 40 degrees C = 313.15 K , and -40 degrees C = 233.15 K.
-
azboy1910

- Exalted Member

- Posts: 146
- Joined: Sat Nov 03, 2018 2:19 pm
- Division: B
- Pronouns: He/Him/His
- Has thanked: 117 times
- Been thanked: 62 times
Re: Density Lab B
1. If the radius of x atom is 70.0 pm, what is the total volume of 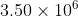 of these atoms in
of these atoms in 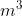 ?
?
2. Calculate the ppb of a 10.0% salt by mass saltwater soultion.
3. The temperature of a gas is at 40 K at the moment and currently has a volume of 13.3 L. The temperature of the gas is increased by the same temperature of the boiling point of water. Calculate the new volume of the gas in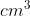 .
.
2. Calculate the ppb of a 10.0% salt by mass saltwater soultion.
3. The temperature of a gas is at 40 K at the moment and currently has a volume of 13.3 L. The temperature of the gas is increased by the same temperature of the boiling point of water. Calculate the new volume of the gas in
-
YellowMamba
- Member

- Posts: 15
- Joined: Wed Sep 30, 2020 12:57 pm
- Division: B
- State: KS
- Has thanked: 0
- Been thanked: 0
Re: Density Lab B
1. Assuming the atom is a perfect sphere: 0.0010262536 m3 5.0286426e^-24
2. 100,000,000 ppb
3. 46550 cm3
Last edited by YellowMamba on Thu Oct 08, 2020 7:14 pm, edited 5 times in total.
-
azboy1910

- Exalted Member

- Posts: 146
- Joined: Sat Nov 03, 2018 2:19 pm
- Division: B
- Pronouns: He/Him/His
- Has thanked: 117 times
- Been thanked: 62 times
Re: Density Lab B
So here's what I got, please feel free to tell me if you disagree:YellowMamba wrote: ↑Thu Oct 08, 2020 10:13 am1. Assuming the atom is a perfect sphere: 0.0010262536 m3 2. 100,000,000 ppb 3. 46550 cm3
Last edited by azboy1910 on Fri Oct 09, 2020 9:37 am, edited 1 time in total.
-
YellowMamba
- Member

- Posts: 15
- Joined: Wed Sep 30, 2020 12:57 pm
- Division: B
- State: KS
- Has thanked: 0
- Been thanked: 0
Re: Density Lab B
Here’s my work for 1 and 3. Correct me if I am wrong:
1: 70 picometers = 7e^-11 Volume of a sphere = 4/3(pi)(r 4/3(pi)(3.43e^-31) = approximately 1.436755e^30 That is the volume of one atom. 1.436755e^-30(3.50)(10^6) = 5.0286426e^-24 I recalculated and got 5.0286426e^-24. I guess I forgot to cube the radius length. 3: 40K and 13.3L to 140K and xL By Charles’s Law I got (13.3)(140) = 40x Solve for x: 1862 = 40x 46.55 = x This is how many liters. To convert to cm3 1L = 1000mL = 1000cm3 So I got 46550 cubic cm.
Last edited by YellowMamba on Thu Oct 08, 2020 7:14 pm, edited 3 times in total.


