Density Lab B
-
SciOly0720
- Member

- Posts: 2
- Joined: March 21st, 2020, 7:05 pm
- Division: B
- State: PA
- Has thanked: 2 times
- Been thanked: 0
Re: Density Lab B
Ok, I did not realize that you were asking for the number of atoms in a mole; I thought you were asking in a molecule.
Q1) In Boyle's Law, what must be kept constant?
Q2) Re-order the following substances in increasing order of density: Aluminum, Mercury, Steel.
Q3) In N/kg, what is the force of gravity on the Earth?
Q1) In Boyle's Law, what must be kept constant?
Q2) Re-order the following substances in increasing order of density: Aluminum, Mercury, Steel.
Q3) In N/kg, what is the force of gravity on the Earth?
-
knightmoves
- Member

- Posts: 599
- Joined: April 26th, 2018, 6:40 pm
- Has thanked: 4 times
- Been thanked: 104 times
Re: Density Lab B
If you just specify "a mole of hydrogen" then than would usually mean a mole of normal diatomic hydrogen gas (azboy1910 wrote: ↑August 12th, 2020, 1:31 pm 1. I apologize if this question was confusing and not worded properly. Here, I was looking for for the amount of atoms (or particles) in a single mole of hydrogen. Therefore, the answer isparticles, as shown below.
- 1 Hydrogen atom1 mole of Hydrogen
Avagadro's Number =
particles
- azboy1910
- Exalted Member

- Posts: 146
- Joined: November 3rd, 2018, 2:19 pm
- Division: B
- Pronouns: He/Him/His
- Has thanked: 117 times
- Been thanked: 62 times
- Contact:
Re: Density Lab B
Here I was talking about monoatomic hydrogen gas, which I forgot to specify. I realize I was not being specific enough and know I need to look at my question first to see if it makes sense before I post it.knightmoves wrote: ↑August 14th, 2020, 3:10 pmIf you just specify "a mole of hydrogen" then than would usually mean a mole of normal diatomic hydrogen gas (azboy1910 wrote: ↑August 12th, 2020, 1:31 pm 1. I apologize if this question was confusing and not worded properly. Here, I was looking for for the amount of atoms (or particles) in a single mole of hydrogen. Therefore, the answer isparticles, as shown below.
- 1 Hydrogen atom1 mole of Hydrogen
Avagadro's Number =
particles
). So that would have
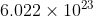
molecules, or
hydrogen atoms.
-
knightmoves
- Member

- Posts: 599
- Joined: April 26th, 2018, 6:40 pm
- Has thanked: 4 times
- Been thanked: 104 times
- azboy1910
- Exalted Member

- Posts: 146
- Joined: November 3rd, 2018, 2:19 pm
- Division: B
- Pronouns: He/Him/His
- Has thanked: 117 times
- Been thanked: 62 times
- Contact:
Re: Density Lab B
I haven't really looked into this all that much (maybe I should), but I suppose it doesn't occur naturally and most likely goes through a certain process if it's rare.knightmoves wrote: ↑August 20th, 2020, 9:45 amUnder what conditions do you think monatomic Hydrogen exists?
Who is online
Users browsing this forum: No registered users and 0 guests

